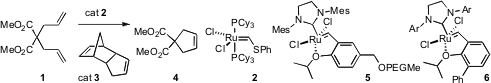
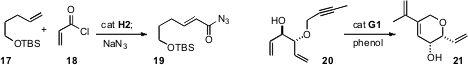The Fischer carbene 2 at 0.5 mol % gives only 12% conversion of 1 to 4 after 18 hours. Debra J. Wallace of Merck Process showed(Adv. Synth. Catal. 2009, 351, 2277.DOI: 10.1002/adsc.200900301) that addition ofa catalytic amount of the inexpensive 3 activated 2, leading to 95%conversion of 1 to 4 after 18 hours. Shazia Zaman of the Universityof Canterbury and Andrew D. Abell of the University of Adelaide found(Tetrahedron Lett. 2009, 50, 5340.DOI: 10.1016/j.tetlet.2009.07.022)that the catalyst 5, incorporating a polyethylene glycol (PEG) chain, was readily recovered in active form by simple extraction, and could be re-used at least five times. 1538623-41-4 Formula Lenalidomide-Br Chemscene Zhu Yinghuai of the Institute of Chemical and Engineering Sciences, Singapore(Adv. Synth. Catal. 2009, 351, 2650.DOI: 10.1002/adsc.200900370)and Chao Che, Zhen Yang and Biwang Jiang of Peking University(Chem. Commun. PMID:34856019 2009, 5990.DOI: 10.1039/b911999j),independently of each other, described the preparation of Ru complexes such as5 bound to magnetic nanoparticles. The catalysts were easily recycled and reused, leaving < 4 ppm Ru in the product.
Reto Dorta of the University of Zurich reported(J. Am. Chem. Soc. 2009, 131, 9498.DOI: 10.1021/ja903554v)that the complex 6 (Ar = 2, 7-diisopropylnaphthyl) was a separable mixtureof syn and anti isomers. The very reactive anti isomer at 50 ppm converted neat1 into 2 in two hours at room temperature.
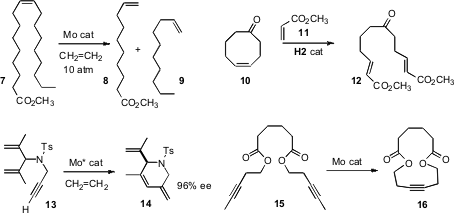
Richard R. Schrock of MIT devised(J. Am. Chem. Soc. 2009, 131, 10840.DOI: 10.1021/ja904786y)an efficient Mo catalyst for a long-sought transformation, the ethenolysis of long-chain alkenes such as 7. Robert A. Stockman of the University of Nottingham developed(Chem. Commun. 2009, 4399.DOI: 10.1039/b906301c)a related Ru-catalyzed procedure, cross-metathesisring opening with methylacrylate 11. Amir A. Hoveyda of Boston College, a co-author on the Schrock paper, used(J. Am. Chem. Soc. 2009, 131, 10652.DOI: 10.1021/ja904098h)a very similar Mo catalyst for the rapid cross metathesis of an alkyne with ethene, leading after subsequentring closing metathesis to products such as 14. Alois Fürstner of the Max-Planck-Institut, Mülheim described(J. Am. Chem. Soc. 2009, 131, 9468.DOI: 10.1021/ja903259g)a well-characterized Mo nitride complex that efficiently catalyzed the conversionof 15 into 16.
Samir Bouzbouz of the Université de Rouen and Janine Cossy of ESPCI Paris established(Org. Lett. 2009, 11, 5446.DOI: 10.1021/ol9021386)conditions for the metathesis of alkenes with the linchpin 18. Exposure ofthe crude acid chloride from 17 to a nucleophile led directly to the coupledproduct 19. Bernd Schmidt of the Universität Potsdam observed(J. Org. Chem. 2009, 74, 9237.DOI: 10.1021/jo9018649)the selective cyclization of 20 to 21. This selectivity may be due to free allylic alcohols being more reactive under metathesis conditions than are allylic ethers.
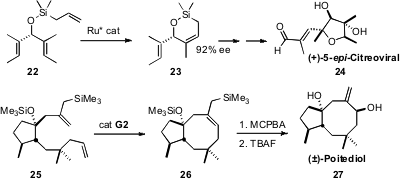
Of the many recent applications of alkene and alkyne metathesis to target-directed synthesis, two particularly stand out. Timothy W. Funk of Gettysburg College cyclized(Org. Lett. 2009, 11, 4998.DOI: 10.1021/ol901853t)the prochiral 22 with an enantiomerically-pure Ru complex. The high ee product 23 was carried over several steps to (+)-5-epi-citreoviral(24).
Christopher D. Vanderwal of the University of California, Irvine showed(J. Am. Chem. Soc. 2009, 131, 15090.DOI: 10.1021/ja906241w)that the second-generation Grubbs catalyst was competent to cyclize 25 tothe trisubstituted cyclooctene 26. Face-selectiveepoxidation followedby desilyation completed the synthesis of (±)-poitediol (27).