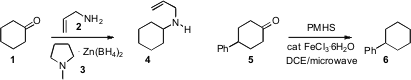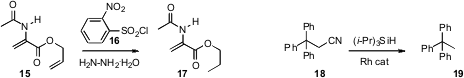Heshmatollah Alinezhad of Mazandaran University, Iran developed(Tetrahedron Lett. 2009, 50, 659.DOI: 10.1016/j.tetlet.2008.11.102)the reagent 3, a white powder that is stable for many months, as ahydride donor for the reductive amination of aldehydes and ketones.Jean-Marc Campagne of the Institut Charles Gephardt Montpellier established(Synlett 2009, 276.DOI: 10.1055/s-0028-1087664)a simple microwave protocol for reducing aldehydes and ketones to the correspondinghydrocarbonsthat looks general enough to become the method of choice for this important transformation.
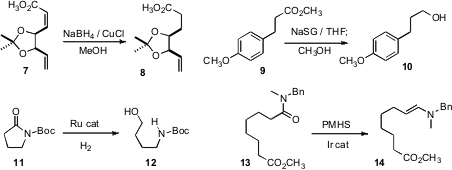
Joanne E. Harvey of Victoria University of Wellington, in the course of a total synthesis of Aigialomycind D, observed(J. 4-(Vinylsulfonyl)benzoic acid Chemical name Org. Chem. 2009, 74, 2271.DOI: 10.1021/jo802561s)that despite the high reactivity of the monosubstituted alkene of 7, theconjugated alkene could be selectively reduced. Brian S. Bodnar of SiGNa Chemistry described(J. Org. Chem. PMID:23290930 Price of 248274-16-0 2009, 74, 2598.DOI: 10.1021/jo802778z) a simple procedure for the reduction of an ester such as 9 to the alcohol10, using Na dispersed on silica gel. Takao Ikariya of the Tokyo Institute of Technology designed(Angew. Chem. Int. Ed. 2009, 48, 1324.DOI: 10.1002/anie.200805307)a Ru complex for the hydrogenation of N-acylsulfonamides and N-acylcarbamates such as 11 to the corresponding alcohol, e.g. 12.
Remarkably, Hideo Nagashima of Kyushu University demonstrated(Chem. Commun. 2009, 1574.DOI: 10.1039/b821317h)that even in the presence of the ester, the amide of 13 could be selectively reduced to the enamine 14. The enamine could be hydrolyzed to the aldehyde or reduced to the amine, but it is also an activated intermediate, for instance forMichael addition to ethyl acrylate or methyl vinyl ketone.
Diimide (HN=NH) is a useful reagent for selective reduction, as illustrated by theconversion of 15 to 17. David R. Carbery of the University of Bath devised(J. Org. Chem. 2009, 74, 3186.DOI: 10.1021/jo900237y)a convenient procedure for the in situ generation of diimide from 16 and hydrazine hydrate.
The reductive cleavage of tertiarynitriles to the corresponding hydrocarbon under dissolving metal conditions has been known for some time(J. Org. Chem. 1996, 61, 4219.DOI: 10.1021/jo960218x).Reduction of secondary nitriles required more forcing conditions, with K metal and crown ether(Tetrahedron Lett. 1985, 26, 6103.DOI: 10.1016/S0040-4039(00)95137-2).Now, Mamoru Tobisu and Naoto Chatani of Osaka University have uncovered(J. Am. Chem. Soc. 2009, 131, 3174.DOI: 10.1021/ja810142v)a Rh catalyst that effects smooth reductive cleavage of primary, secondary and tertiary nitriles.
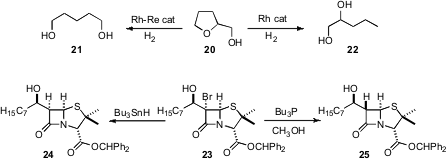
Tetrahydrofurfuryl alcohol 20 is an important biomass-derived feedstock. Keiichi Tomishige of the University of Tsukuba reported(Chem. Commun. 2009, 2035.DOI: 10.1039/b822942b)conditions for the selective cleavage of 20 to, respectively, 21 or 22.
Shahriar Mobashery of the University of Notre Dame demonstrated(Org. Lett. 2009, 11, 2515.DOI: 10.1021/ol900668k)selectivity of a different sort in the debromination of 23.Free radical reduction gave the diastereomer 24, while ionic reductiondelivered 25. In a complemenary development, Maurice Brookhartof the University of North Carolina described(Adv. Synth. Catal. 2009, 351, 175.DOI: 10.1002/adsc.200800528)an Ir catalyst for the Et3SiH reduction ofhalides to hydrocarbons.