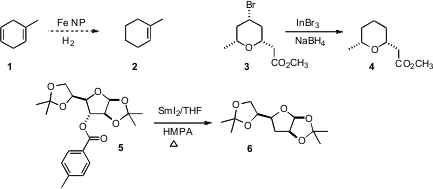
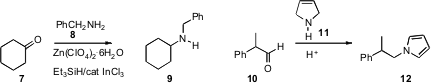
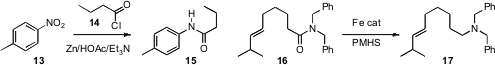
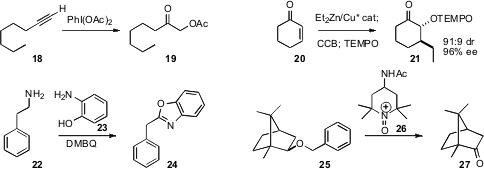
Johannes G. de Vries of DSM Pharmaceuticals prepared
(Chem. Commun. 2009, 3747.
DOI: 10.1039/b820048c)
Fe nanoparticles that selectively mediated the hydrogenation of
Z alkenes and not trisubstituted alkenes. This should allow
the conversion of 1 to 2. In the course of a synthesis
(Tetrahedron Lett. 2009, 50, 4368.
DOI: 10.1016/j.tetlet.2009.05.053)
of centrolobine, Teck-Peng Loh of Nanyang Technological University employed an
elegant protocol for the reduction of the secondary bromide 3. Price of 14871-41-1 István Markó
of the Université catholique de Louvain observed
(Tetrahedron 2009, 65, 10930.
DOI: 10.1016/j.tet.2009.09.111)
that toluates such as 5 can be reduced smoothly with
SmI2 to the
corresponding C-H.
Dan Yang of the University of Hong Kong devised
(Org. Lett. 2009, 11, 3302.
DOI: 10.1021/ol901111g)
a triethylsilane-based procedure for the reductive amination of aldehydes
and ketones such as 7. PMID:23381601 Jon A. Tunge of the University of Kansas developed
(J. Am. 1222174-93-7 Chemical name Chem. Soc. 2009, 131, 16626.
DOI: 10.1021/ja907357g)
a complementary protocol for the conversion of an aldehyde or ketone to the
protected amine 12. Mark T. Hamann of the University of Mississippi established
(Tetrahedron Lett. 2009, 50, 3901.
DOI: 10.1016/j.tetlet.2009.04.061)
that a nitroaromatic 13 could be reduced in the presence of an acid chloride
14 to deliver the amide 15 directly. Matthias Beller of the Universität Rostock
(Angew. Chem. Int. Ed. 2009, 48, 9507.
DOI: 10.1002/anie.200904677)
and Hideo Nagashima of Kyushu University
(Angew. Chem. Int. Ed. 2009, 48, 9511,
DOI: 10.1002/anie.200905025;
J. Am. Chem. Soc. 2009, 131, 15032,
DOI: 10.1021/ja9055307)
reported parallel investigations of the silane-based
reduction of an amide 16
to the amine 17.
Xue-Long Hou of the Shanghai Institute of Organic Chemistry demonstrated
(Tetrahedron Lett. 2009, 50, 5578.
DOI: 10.1016/j.tetlet.2009.07.081)
that a terminal alkyne 18 could be oxidized to the α-acetoxy ketone
19. Philippe Renaud
of the Universität Bern and Armido Studer of Westfälische-Wilhelms-Universität established
(Angew. Chem. Int. Ed. 2009, 48, 6037.
DOI: 10.1002/anie.200902242)
that both zinc enolates and silyl enol ethers could combine with chlorocatechol borane followed
by
TEMPO to give the α-oxygenated ketone. Stephen P. Marsden of the University of Leeds devised
(Tetrahedron Lett. 2009, 50, 6106.
DOI: 10.1016/j.tetlet.2009.08.042)
a protocol for oxidizing a primary amine 18 to the benzoxazole
24, which has the oxidation state of the carboxylic acid. James M. Bobbitt and
William F. Bailey of the University of Connecticut developed
(J. Org. Chem. 2009, 74, 9524.
DOI: 10.1021/jo902144b)
the oxammonium salt 26 for the direct oxidation of
benzyl ethers
to ketones and carboxylic acids.
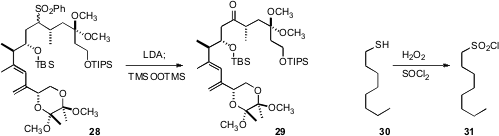
Rich G. Carter of Oregon State University effected
(Org. Biomol. Chem. 2009, 7, 4582.
DOI: 10.1039/b916744g)
oxidation of the complex sulfone 28 to the ketone 29. Kiumars Bahrami
and Mohammad M. Khodaei of Razi University found
(J. Org. Chem. 2009, 74, 9287.
DOI: 10.1021/jo901924m)
that oxidation of a thiol 30 to the sulfonyl chloride 31 could be followed by
direct coupling to an amine to yield the
sulfonamide.