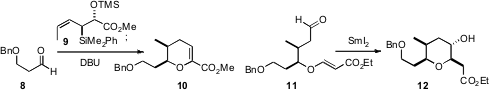SC-Δ13-9-IsoF (Taber), Brevisamide (Panek, Lindsley, Ghosh), Gambierol (Mori)
The challenge of controlling the relative and absolute configuration of highly
substituted cyclic
ether-containing natural products continues to stimulate the
development of new synthetic methods. Masahiro Murakami of Kyoto University showed
(J. Iodo-PEG3-N3 Price Org. Chem. 2009, 74, 6050.
DOI: 10.1021/jo900987w)
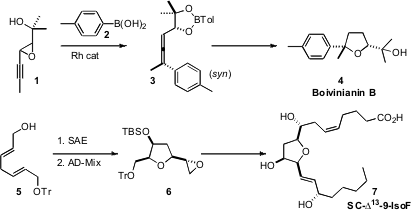
that Rh-mediated addition of an
aryl boronic acid to 1 proceeded with high syn
diastereocontrol, to give 3. This set the stage for Au-mediated
rearrangement, leading to 4. 1-(Difluoromethyl)-4-iodo-1H-pyrazole Data Sheet We found
(J. Org. Chem. 2009, 74, 5516.
DOI: 10.1021/jo900767x)
that asymmetric epoxidation of 5 followed by
exposure to
AD-mix could be used to prepare each of the four diastereomers of 6. PMID:27641997 We
carried 6 on the isofuran 7, using a stereodivergent strategy that
allowed the preparation of each of the 32 enantiomerically-pure diastereomers of
the natural product.
Following up on the synthesis of Brevisamide 16 described
(![]()
2009, November 16) by Kazuo Tachibana of the University of Tokyo, three groups
reported alternative total syntheses. James S. Panek of Boston University prepared
(Org. Lett. 2009, 11, 4390.
DOI: 10.1021/ol901801h)
the cyclic ether of 16 by addition of the enantiomerically-pure silane 9
to 8. Craig W. Lindsley of Vanderbilt University used
(Org. Lett. 2009, 11, 3950.
DOI: 10.1021/ol9015755)
SmI2 to effect the cyclization of 11 to 12.
Arun K. Ghosh of Purdue University employed
(Org. Lett. 2009, 11, 4164.
DOI: 10.1021/ol901691d)
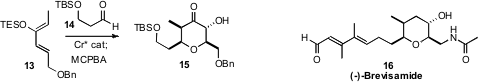
an enantiomerically-pure Cr catalyst to direct the absolute
configuration in the hetero
Diels-Alder addition of 14 to 13.
Rubottom oxidation of the enol ether so formed led to the α-hydroxy ketone
15.
Yuji Mori of Meijo University described
(Org. Lett. 2009, 11, 4382.
DOI: 10.1021/ol9017408)
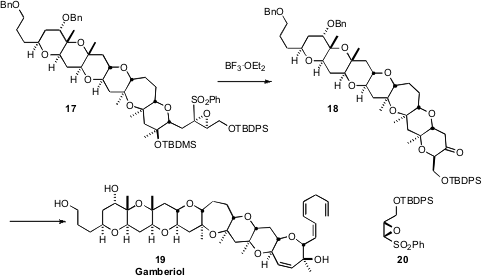
the total synthesis of the Gambierdiscus toxicus ladder ether Gambierol (19).
A key strategy, used repeatedly through the sequence, was the exo cyclization of an
epoxy sulfone, illustrated by the conversion of 17 to 18. The epoxy sulfones
were prepared by alkylating the anions derived from preformed epoxy sulfones
such as 20.