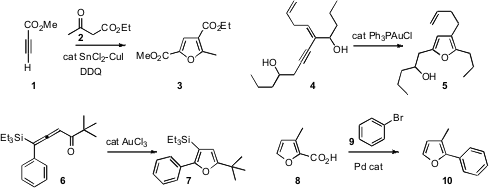
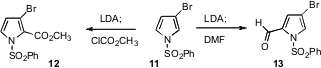Huanfeng Jiang of the South China University of Technology showed
(J. Org. 5-Bromo-2-(tert-butyl)pyridine Purity Chem. 2010, 75, 966.
DOI: 10.1021/jo902375k)
that an alkynoate 1 could be condensed with a 1,3-dicarbonyl compound 2 to give,
under oxidizing conditions, the
furan 3. Phil Ho Lee of Kangwon National University found
(Tetrahedron Lett. 2010, 51, 1899.
DOI: 10.1016/j.tetlet.2010.02.026)
that the enyne 4 cyclized smoothly to the furan 5. Yahong Li of Suzhou
University and Vladimir Gevorgyan of the University of Illinois, Chicago demonstrated
(J. PMID:23329319 Am. Chem. Soc. 2010, 132, 7645.
DOI: 10.1021/ja910290c)
that the cyclization of 6 proceeded with silyl migration, to give 7.
François Bilodeau and Pat Forgione of Boehringer Ingelheim (Canada) optimized
(J. Org. Formula of 1639-66-3 Chem. 2010, 75, 1550.
DOI: 10.1021/jo9022793)
the Pd-mediated decarboxylative coupling of a furoic acid 8 with 9 to give 10. This
protocol also worked well with pyrrole carboxylic acids.
In another transformation of a preformed
pyrrole, Masatomo Iwao of Nagasaki University observed
(Org. Lett. 2010, 12, 2734.
DOI: 10.1021/ol100810c)
that in the presence of LDA/diisopropylamine, the initially-formed 2-anion from
the deprotonation of 11 gave the 2-product 12 with more reactive
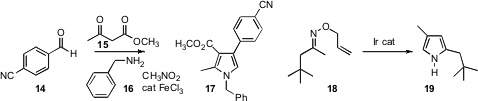
electrophiles, but the 5-product 13 with less reactive electrophiles. Umasish Jana of Jadavpur University developed
(J. Org. Chem. 2010, 75, 1674.
DOI: 10.1021/jo902661y)
a route to more highly substituted pyrroles such as 17 using the remarkable four-component
coupling of 14, 15 and 16 with nitromethane, the carbon of which was incorporated
in the product. Laura L. Anderson, also of the University of Illinois, Chicago, designed
(Org. Lett. 2010, 12, 2290.
DOI: 10.1021/ol100659q)
a clever approach to pyrroles, based on the Ir-catalyzed rearrangement of O-allyl oximes such as 18.
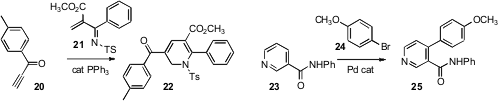
Xiaofeng Tong of the East China University of Science and Technology reported
(Chem. Commun. 2010, 312.
DOI: 10.1039/B915825A)
the condensation of 20 with 21 to give the dihydropyridine 22.
Base-mediated elimination of sulfinate could convert 22 into
the pyridine. Jin-Quan Yu of Scripps/La Jolla found
(Angew. Chem. Int. Ed. 2010, 49, 1275.
DOI: 10.1002/anie.200906104)
that Pd-mediated activation of the nictotinamide 23 proceeded with
high regioselectivity, leading to 25.
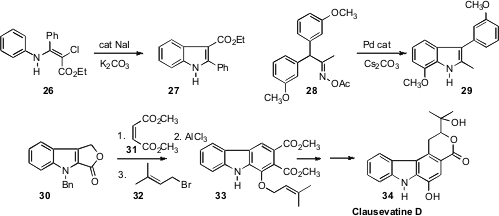
Zhiping Li of Remnin University of China demonstrated
(J. Org. Chem. 2010, 75, 4636.
DOI: 10.1021/jo100796s)
that the chloroenamine 26 cyclized to the
indole 27 on exposure to NaI.
John F. Hartwig of the University of Illinois devised
(J. Am. Chem. Soc. 2010, 132, 3676.
DOI: 10.1021/ja100676r)
a Pd catalyst for the cyclization of the oxime acetate 28 to the indole 29.
Dipakranjan Mal of the Indian Institute of Technology, Kharagpur took advantage
(Chem. Commun. 2010, 4411.
DOI: 10.1039/C0CC00527D )
of the Sammes carboline construction in the
conversion of 30 to Clausevatine D (34).