Keiji Maruoka of Kyoto University found
(Org. 8-Bromoquinazoline-2,4-diol Purity Lett. 2010, 12, 1668.
DOI: 10.1021/ol100104v)
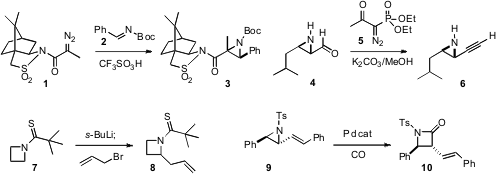
that the diazo amide 1 derived from the Oppolzer sultam condensed
with the imine 2 to give the aziridine 3 with high stereocontrol.
Andrei K. Yudin of the University of Toronto observed
(Angew. Chem. Int. Ed. 2010, 49, 1607.
DOI: 10.1002/anie.200906066)
that the unprotected aziridine aldehyde 4,
which exists as a mixture of dimers, condensed smoothly with the
Ohira reagent (5)
to give the alkynyl aziridine 6.
David M. Hodgson of the University of Oxford successfully
(Angew. PMID:24324376 Chem. Int. Ed. 2010, 49, 2900.
DOI: 10.1002/anie.201000058)
deprotonated the azetidine thioamide 7 to give, after allylation,
the azetidine 8. Varinder K. Aggarwal of the University of Bristol devised
(Chem. Commun. 2010, 267.
DOI: 10.1039/B920564K)
a Pd catalyst for the cyclocarbonylation of
an alkenyl aziridine 9 to give the
β-lactam 10.
Iain Coldham of the University of Sheffield used
(J. Price of 4-Bromothiazolo[5,4-c]pyridin-2-amine Org. Chem. 2010, 75, 4069.
DOI: 10.1021/jo100415x)
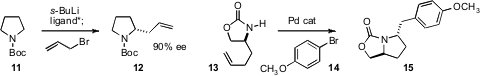
the ligand they had developed to effect enantioselective allylation of the
pyrrolidine derivative 11. The corrresponding
piperidine worked as well. John P.
Wolfe of the University of Michigan established
(Org. Lett. 2010, 12, 2322.
DOI: 10.1021/ol1006828)
that the Pd-mediated cyclization of 13 to 15 could be effected with high
diastereocontrol. Christopher G. Frost of the University of Bath optimized
(Angew. Chem. Int. Ed. 2010, 49, 1825.
DOI: 10.1002/anie.200907067)
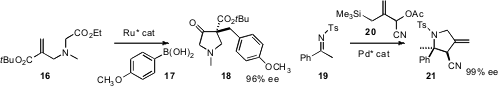
the tandem Ru-mediated conjugate addition/cyclization of 16, to give 18
in high ee. Barry M. Trost of Stanford University extended
(J. Am. Chem. Soc. 2010, 132, 8238.
DOI: 10.1021/ja102102d)
their studies of trimethylenemethane cycloaddition to the ketimine 19,
leading to the substituted pyrrolidine 21 in high ee.
Pher G. Andersson of Uppsala University optimized
(J. Am. Chem. Soc. 2010, 132, 8880.
DOI: 10.1021/ja103901e)
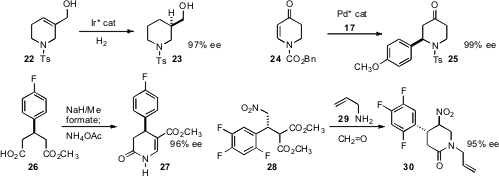
an Ir catalyst for the enantioselective hydrogenation of readily-prepared tetrahydropyridines
such as 22. Min Shi of the Shanghai Institute of Organic Chemistry devised
(J. Org. Chem. 2010, 75, 3935.
DOI: 10.1021/jo1006224)
a Pd catalyst for enantioselective conjugate addition to the prochiral pyridone 24.
Xiaojun Huang of Roche Palo Alto prepared
(Tetrahedron Lett. 2010, 51, 1554.
DOI: 10.1016/j.tetlet.2010.01.049)
the monoacid 26 by enantioselective methanolysis of the anhydride. Selective
formylation of the ester led to the pyridone 27. Feng Xu of Merck Rahway established
(J. Org. Chem. 2010, 75, 1343.
DOI: 10.1021/jo902573q)
the stereogenic center of 28 by
enantioselective conjugate addition of malonate to the β-nitrostyrene. Mannich
condensation with formaldehyde and the amine 29 then delivered 30.
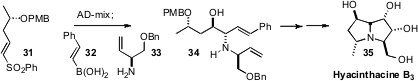
It is also important to develop strategies for the preparation of polycyclic alkaloids
such as Hyacinthacine B3 (35). Stephen G. Pyne of the University of Wollongong assembled
(Chem. Commun. 2010, 713.
DOI: 10.1039/B918233K)
the intermediate 34 for the synthesis of 35 by Petasis condensation of
32 and 33 with the α-hydroxy aldehyde
prepared by asymmetric dihydroxylation of 31.