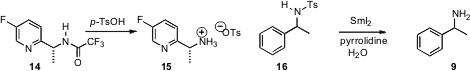Masato Kitamura of Nagoya University investigated
(Chem. Lett. 2009, 38, 188.
DOI: 10.1246/cl.2009.188)
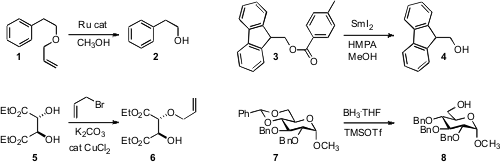
the Ru-mediated deprotection of allyl ethers such as 1. The same catalyst
was effective for the preparation of allyl ethers from the alcohol 2 and allyl
alcohol. István E. Formula of 76947-02-9 Markó of the Université Catholique de Louvain showed
(Org. Lett. 2009, 11, 2752.
DOI: 10.1021/ol900828x)
that SmI2 effected the reductive cleavage of an aryl ester 3,
liberating the alcohol 4.
Osamu Onomura of Nagasaki University found
(Tetrahedron Lett. 2009, 50, 1466.
DOI: 10.1016/j.tetlet.2009.01.063)
that catalytic CuCl2 mediated the selective
monoallylation of symmetrical diols such as 5. PMID:23319057 Péter Fügedi of the
Hungarian Academy of Sciences, Budapest observed
(Tetrahedron Lett. 2009, 50, 2914.
DOI: 10.1016/j.tetlet.2009.03.194)
that TMSOTf catalyzed the selective reduction of 7
to the benzyl ether. BH3•NMe2 delivered the opposite
regioisomer.
Direct amination of an ester has been a long-sought transformation. Vladimir
B. Imidazo[1,2-a]pyridine-8-carbaldehyde Order Birman of Washington University found
(Org. Lett. 2009, 11, 1499.
DOI: 10.1021/ol900098q)
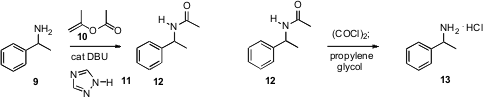
that 1,2,4-triazole 11 in combination with DBU was an effective
catalyst for this reaction. Unactivated esters required higher reaction
temperatures.
Deprotection of amides often requires vigorous conditions, and the product
free amines can be challenging to handle. Stefan G. Koenig of Sepracor Chemical
Process devised
(Org. Lett. 2009, 11, 433.
DOI: 10.1021/ol802482d)
a simple protocol for in situ formation and hydrolysis of the imidoyl chloride from 12
that delivered the amine hydrochloride 13 directly. Remarkably,
benzamides are stable under these conditions. Hongmei Li of Merck Process devised
(Tetrahedron Lett. 2009, 50, 1010.
DOI: 10.1016/j.tetlet.2008.12.050)
a related procedure, heating the more reactive
trifluoroacetamide 14 with
stoichiometric p-toluenesulfonic acid, and isolating the product as the
p-toluenesulfonate salt 15.
There has been a reluctance to use
sulfonamide protecting groups, as they have been thought
to be difficult to remove. Göran Hilmersson of the University of Gothenburg established
(Org. Lett. 2009, 11, 503.
DOI: 10.1021/ol802243d)
that SmI2 instantaneously deprotected 16.
Daniel E. Falvey of the University of Maryland designed
(J. Org. Chem. 2009, 74, 3894.
DOI: 10.1021/jo900182x)
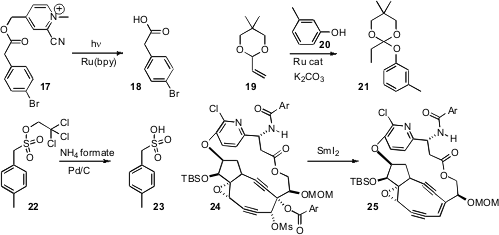
the benzyl ester 17, that was readily released under photolysis.
Stanislaw Krompiec of the University of Silesia described
(Tetrahedron Lett. 2009, 50, 1193.
DOI: 10.1016/j.tetlet.2008.12.110)
what appears to be a general strategy for the preparation of ortho esters such as 21.
To incorporate a sulfonic acid in a synthetic target, it is usually necessary
to bring it along in protected form, then release the free sulfonic acid at the
end of the synthesis. Scott D. Taylor of the University of Waterloo devised
(J. Org. Chem. 2009, 74, 3583.
DOI: 10.1021/jo900122c)
the trichloroethyl ester 22 for this purpose.
Masahiro Hirama of Tohoku University employed
(Angew. Chem. Int. Ed. 2009, 48, 1110.
DOI: 10.1002/anie.200805518)
the ester mesylate 24 as a protected form of the alkene 25. Note that the sensitive 25 was
stable to the SmI2 reduction.