Enantioselective target-directed synthesis, which is important both for single-enantiomer pharmaceuticals and for natural product total synthesis, depends on the ability to form carbon-carbon bonds with absolute stereocontrol. Ethyl 4-methyl-1H-pyrrole-2-carboxylate Formula An ideal method would be readily available, easy to practice, and proceed with high stereocontrol. 2,4-Dichloro-6-ethylpyrimidine structure In this column, we conclude our review of the most prominent recent developments. PMID:35345980 (Part one)
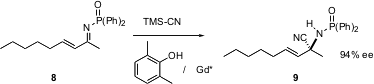
Asymmetric conjugate addition is a powerful method for the construction of ternary stereogenic centers. Erick Carreira of the ETH-Hönggerberg, Zürich reports (Org. Lett. 2004,6, 2281.DOI: 10.1021/ol0491585)the chiral-auxiliary mediated conjugate addition of alkynes to alkylidene malonate derivatives such as1, to give, after hydrolysis and decarboxylation, the enantiomerically-enriched acid3.
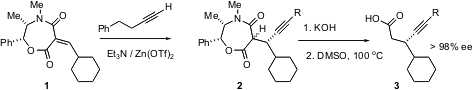
Enantioselective conjugate addition can also be carried out with cyclic enones. Shuichi Oi and Yoshio Inoue of Tohoku University in Sendai report (Tetrahedron Lett. 2004, 45, 5051.DOI: 10.1016/j.tetlet.2004.04.171)that a BINAP complex of Rh catalyzes the enantioselective conjugate addition of alkenyl Zr species such as5, to give 7 in high enantiomeric excess. Alkenyl Zr species such as 5 are readily prepared by hydridozirconation of alkynes. It is particularly important that addition of TMS-Cl to the reaction mixture at the end of the conjugate addition leads cleanly to the enol ether6.
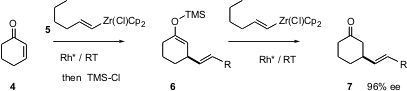
The asymmetric Strecker reaction, the addition of cyanide to an aldehyde imine, is one of the better ways to prepare α-amino acids. Masakatsu Shibasaki and Motomu Kanai of the University of Tokyo report (Tetrahedron Lett. 2004, 45, 3147,DOI: 10.1016/j.tetlet.2004.02.082; 3153,DOI: 10.1016/j.tetlet.2004.02.077)that use of a catalyst prepared from Gd isopropoxide and a glucose-derived phosphine oxide allows the asymmetric Strecker reaction to be carried out onketone-derived imines such as8. Aryl alkyl ketones work well, as do alkyl methyl ketones. It is especially noteworthy that imines such as 8 derived from long-chain α,β-unsaturated ketones also give addition with high ee.