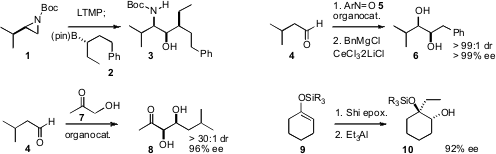
Varinder K. Aggarwal of the University of Bristol showed(Angew. Chem. Int. Ed. 2009, 48, 1149.DOI: 10.1002/anie.200805272)that condensation of a boronic ester 2 with a metalatedaziridine 1 led, after oxidation, to the defined aminoalcohol 3. Hisashi Yamamoto of the University of Chicago developed(Angew. Chem. Int. Ed. 2009, 48, 3333.DOI: 10.1002/anie.200900682)conditions for the diastereoselective addition of an organometallic to anα-nitrosylated aldehyde, to give, after reduction, the diol 6.Xiaoyu Wu of Shanghai University and Gang Zhao of the ShanghaiInstitute of Organic Chemistry designed(Adv. Synth. Formula of 1-Hydroxycyclobutanecarbonitrile Catal. 2009, 351, 158.DOI: 10.1002/adsc.200800558)an organocatalyst that mediated the enantioselective addition of hydroxyacetone7 to a range of aldehydes. PMID:25027343 Andrew G. 2-Furanboronic acid Chemscene Myers of Harvard University found(J. Am. Chem. Soc. 2009, 131, 5763.DOI: 10.1021/ja901283q)that trialkylaluminum reagents opened epoxides of enol ethers at the moresubstituted position, delivering protected diols such as 10.
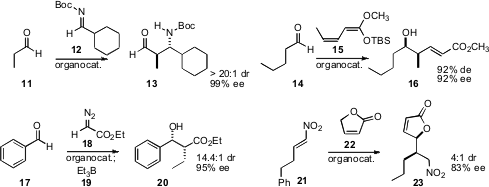
Keiji Maruoka of Kyoto University created(Angew. Chem. Int. Ed. 2009, 48, 1838.DOI: 10.1002/anie.200805628)an organocatalyst for the addition of an aldehyde 11 to an imine 12,to give 13. Markus Kalesse of Leibnitz Universität Hannover showed(Tetrahedron Lett. 2009, 50, 3485.DOI: 10.1016/j.tetlet.2009.03.013)that an organocatalyst could mediate the selective γ-reactivity of 15, leading to 16. Barry M. Trost of Stanford University found(J. Am. Chem. Soc. 2009, 131, 1674.DOI: 10.1021/ja809181m)that an organocatalyst directed the addition of diazoacetate 18to an aldehyde, to give, after further reaction with a trialkylborane,the syn aldol product 19. Professor Trost also demonstrated(J. Am. Chem. Soc. 2009, 131, 4572.DOI: 10.1021/ja809723u)that a related complex mediated the conjugate addition of 22 to 21.
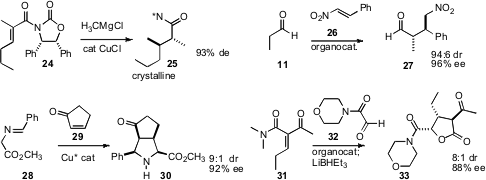
Enantioselective construction of arrays of alkylated stereogeniccenters is a particular challenge. Ji Zhang, then at Pfizer, found(Tetrahedron Lett. 2009, 50, 1167.DOI: 10.1016/j.tetlet.2008.12.111)that the chiral auxiliary of 24 directed both the conjugate addition and thesubsequent protonation, and also allowed the product 25 to be brought to >98%purity by crystallization. Tönis Kanger of Tallinn University of Technology developed(J. Org. Chem. 2009, 74, 3772.DOI: 10.1021/jo900322h)an organocatalyst for the conjugate addition of aldehydes to nitrostyrenes such as26, to give 27. Juan C. Carretero of the Universidad Autónoma de Madrid designed(Org. Lett. 2009, 11, 393.DOI: 10.1021/ol802664m)a Cu catalyst that mediated the long-soughtdipolar cycloaddition of 28 to enones such as 29. Tomislav Rovis of Colorado State University established(Org. Lett. 2009, 11, 2856.DOI: 10.1021/ol901081a)that a triazolinylidene carbene effected conjugate addition of 32 to 31.Reduction proceeded with high diastereocontrol, to deliver 33.
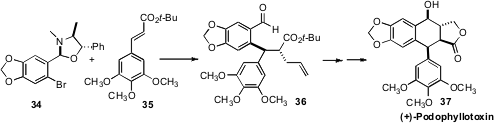
In the course of a synthesis of (+)-Podophyllotoxin (37),Hongbin Zhang of Yunnan University used(Org. Lett. 2009, 11, 597.DOI: 10.1021/ol8026208)a different sort of chiral auxiliary control, an oxazolidine ortho to anaryl lithium, to set the absolute sense of the conjugate addition to 35.Allylation proceeded with high diastereocontrol, and the enantiomerically-pure aminoalcohol director could be recovered and recycled.