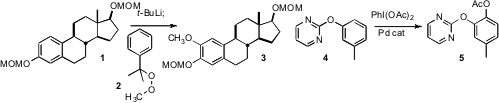
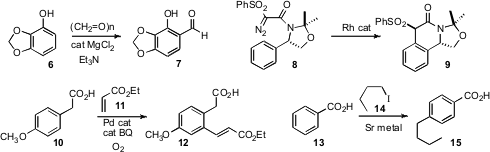
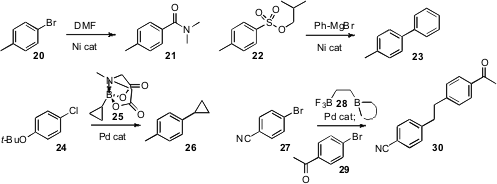
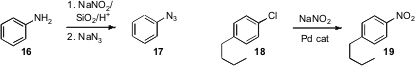Yuqing Hou of Southern Illinois University found
(J. Org. Chem. PMID:24187611 2009, 74, 6362.
DOI: 10.1021/jo901086s)
that the peroxy ether 2 served effectively to directly transfer a methoxy group
to the lithiated 1 to give 3. Wanzhi Chen of Zhejiang University, Xixi Campus, showed
(J. Org. Chem. 2009, 74, 7203.
DOI: 10.1021/jo901316b)
that pyrimidines such as 4, readily prepared from the corresponding phenol, underwent
smooth Pd-catalyzed ortho acetoxylation.
Trond Vidar Hansen of the University of Oslo observed
(Tetrahedron Lett. 2009, 50, 6339.
DOI: 10.1016/j.tetlet.2009.08.101)
that simple electrophilic formylation of phenols such as 6 also proceeded with high
ortho selectivity. Kyung Woon Jung of the University of Southern California optimized
(J. Org. Chem. 86208-18-6 Price 2009, 74, 6231.
DOI: 10.1021/jo9011255)
the Rh catalyst for ortho C-H insertion, converting 8 into 9. Methyl 1H-imidazole-5-carboxylate In stock
Jin-Quan Yu of Scripps/La Jolla devised
(Science 2010, 327, 315.
DOI: 10.1126/science.1182512)
a protocol for carboxy-directed catalytic ortho palladation that allowed subsequent
Heck
coupling, transforming 10 into 11. Norikazu Miyoshi of the University of Tokushima established
(Chem. Lett. 2009, 38, 996.
DOI: 10.1246/cl.2009.996)
that in situ generated strontium alkyls added 1,6 to benzoic acid 13, to give, after
mild oxidative workup, the 4-alkyl benzoic acid 15.
Amin Zarei of Islamic Azad University showed
(Tetrahedron Lett. 2009, 50, 4443.
DOI: 10.1016/j.tetlet.2009.05.049)
that their previously-developed protocol for preparing stable diazonium silica sulfates could be
extended to the preparation of an aryl azide such as 17. Stephen L. Buchwald of MIT developed
(J. Am. Chem. Soc. 2009, 131, 12898.
DOI: 10.1021/ja905768k)
a Pd-mediated protocol for the conversion of aryl chlorides to the corresponding
nitro aromatics. Virgil Percec of the University of Pennsylvania has also reported
(Org. Lett. 2009, 11, 4974.
DOI: 10.1021/ol902155e)
the conversion of an aryl chloride to the borane, and Guy C. Lloyd-Jones has described
(Angew. Chem. Int. Ed. 2009, 48, 7612.
DOI: 10.1002/anie.200903908) the
conversion of phenols to the corresponding thiols.
Kwang Ho Song of Korea University and Sunwoo Lee of Chonnam National University demonstrated
(J. Org. Chem. 2009, 74, 6358.
DOI: 10.1021/jo901065y)
that the Ni-mediated homologation of aryl halides worked with a variety of primary
and secondary formamides. Kwangyong Park of Chung-Ang University observed
(J. Org. Chem. 2009, 74, 9566.
DOI: 10.1021/jo902151h)
that nickel catalysts also mediated the
coupling of Grignard reagents
with the tosylate 22 not in the usual way, but with the C-S bond, to give
23.
Martin D. Burke of the University of Illinois reported
(J. Am. Chem. Soc. 2009, 131, 6961.
DOI: 10.1021/ja901416p)
that the derivative 25 served as a useful slow-release source for otherwise unstable
boronic acids. Gary A. Molander of the University of Pennsylvania took advantage
(Org. Lett. 2009, 11, 2369.
DOI: 10.1021/ol900822j)
of the marked difference in reactivity of the two boranes of 28 to effect sequential coupling.
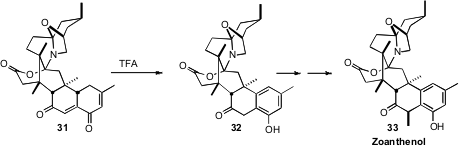
It is sometimes most efficient to construct the aromatic ring. This is
illustrated by the tautomerization of 31 to 32, the key step in the total
synthesis of Zoanthenol (33) reported
(Angew. Chem. Int. Ed. 2009, 48, 8905.
DOI: 10.1002/anie.200904537)
by Keiji Tanino and Masaaki Miyashita of Hokkaido University.