α-Agorafuran
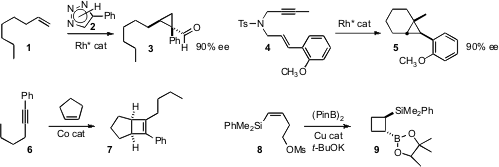
Valery V. Fokin of Scripps/La Jolla extended
(J. Am. Chem. Soc. 1279894-35-7 Purity 2010, 132, 2510.
DOI: 10.1021/ja910187s)
enantioselective Rh-mediated intermolecular
cyclopropanation to α-olefins such as
1. Takahiro Nishimura and Tamio Hayashi of Kyoto University developed
(Angew. Chem. Int. Ed. 2010, 49, 1638.
DOI: 10.1002/anie.200906792)
a procedure for enantioselective intramolecular cyclopropanation, beginning with a
propargyl sulfonamide 4. Gerhard Hilt of Philipps-Universität Marburg established
(Org. Lett. 2010, 12, 1536.
DOI: 10.1021/ol100266u)
that an aryl alkyne 6 could add to
cyclopentene to give
the cyclobutene 7. PMID:23543429 Hajime Ito of Hokkaido University devised
(J. Am. Chem. Soc. 2010, 132, 5990.
DOI: 10.1021/ja101793a)
a protocol for the borylation of an alkenyl silane 8 to give an
intermediate α-lithio silane, that cyclized to the
cyclobutane 9 with high
diastereoconrol. Aryl alkenes worked as well. This same approach could be used
to construct cyclopentanes and
cyclohexanes.
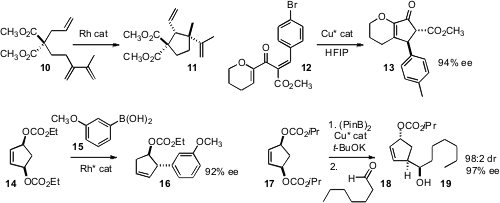
Zhi-Xiang Yu of Peking University showed
(J. Am. 5-Bromo-1,3-thiazole-2-carbaldehyde Chemical name Chem. Soc. 2010, 132, 4542.
DOI: 10.1021/ja100409b)
that the triene 10 cyclized to the cyclopentane 11 with high diastereocontrol.
Yong Tang of the Shanghai Institute of Organic Chemistry optimized
(Angew. Chem. Int. Ed. 2010, 49, 4463.
DOI: 10.1002/anie.200907266)
a Cu catalyst for the enantioselective
Nazarov cyclization of 12 to 13.
The meso bis-carbonates 14 and 16 were prepared by singlet oxygenation of
the inexpensive cyclopentadiene. Mark Lautens of the University of Toronto developed
(J. Org. Chem. 2010, 75, 4056.
DOI: 10.1021/jo100391e)
a protocol for the enantioselective coupling of 14 to an arene boronic acid,
to give the carbonate 15 with high enantiocontrol. Professor Ito devised
(Angew. Chem. Int. Ed. 2010, 49, 560.
DOI: 10.1002/anie.200905993)
a complementary procedure, coupling 16 with bis-pinacolatoborane to give an
intermediate allylborinate, that then added to the aldehyde 18 to give 19
with high stereocontrol. It would be interesting to know if these procedures worked
as well with the meso bis-carbonates derived from cyclohexadiene.
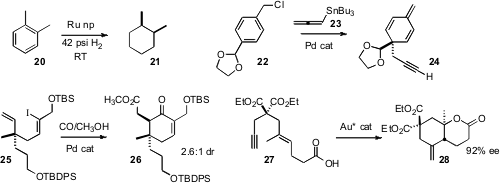
Saim Özkar of Middle East Technical University prepared
(J. Am. Chem. Soc. 2010, 132, 6541.
DOI: 10.1021/ja101602d)
Ru nanoclusters stabilized by a zeolite framework that effected hydrogenation of
20 at near ambient conditions, in contrast to the high pressure and temperature
usually required. Xiujuan Feng and Ming Bao of the Dalian University of Technology showed
(J. Org. Chem. 2010, 75, 2619.
DOI: 10.1021/jo100211d)
that the allenyl stannane 23 coupled with a benzyl chloride 22
to give the cyclohexadiene 24.
Tomoyuki Esumi and Yoshiyasu Fukuyama of Tokushima Bunri University cyclized
(Org. Lett. 2010, 12, 888.
DOI: 10.1021/ol902960n)
the iodide 25 to the ester 26.
F. Dean Toste of the University of California, Berkeley developed
(J. Am. Chem. Soc. 2010, 132, 8276.
DOI: 10.1021/ja103544p)
a gold catalyst for the enantioselective cylization of 27 to 28.
The catalyst also worked for polyenes, delivering the
polycarbocycles with high stereocontrol.
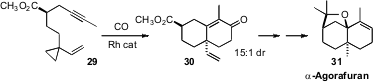
Professor Yu found
(Org. Lett. 2010, 12, 2528.
DOI: 10.1021/ol100625e)
that the alkyne 29 cyclized to 30 with substantial diastereocontrol, setting the
stage for the synthesis of α-Agorafuran (31). It would be easy to prepare alkynes such
as ester 29 in enantiomerically-pure form. The enone 30 would be a versatile
intermediate for the preparation of a variety of polycarbocyclic natural products.