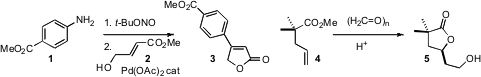
Arene diazonium salts are effective precursors for the Heck reaction. Sandro Cacchi of the Università degli Studi “La Sapienza”, Roma, observed(Synlett 2009, 1277.DOI: 10.1055/s-0028-1088132)that the diazonium salt generated in situ from 1 coupled with 2 to deliverthe butenolide 3. Daniel J. Canney of Temple University established(Tetrahedron Lett. 2009, 50, 5914.DOI: 10.1016/j.tetlet.2009.08.016)conditions for the homologation of an alkenyl ester such as 4 to the homologated lactone 5. Buy(4,5-Dimethoxy-2-nitrophenyl)methanol
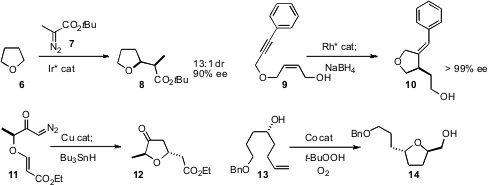
Tsutomu Katsuki of Kyushu University established(J. Am. Chem. Soc. 7-Methoxyisoquinolin-1-ol manufacturer 2009, 131, 14218.DOI: 10.1021/ja9065267)that Ir-mediated C-H insertion converted 6 into 8 with high diastereo-and enantiocontrol. Thomas J. J Müller of the Heinrich-Heine-Universität, Düsseldorf optimized(Adv. Synth. Catal. 2009, 351, 2921.DOI: 10.1002/adsc.200900494)the Rh-mediated enantioselective cycloisomerization of 9 to 10.Santosh J. Gharpure of the Indian Institute of Technology Madras showed(Org. Lett. 2009, 11, 5466.DOI: 10.1021/ol902301h)that the intramolecular cyclopropanation of 11 proceeded with high diasterocontrol. PMID:24670464 Reduction of the intermediatecyclopropane then delivered the cyclicether 12. Brian L. Pagenkopf of the University of Western Ontario optimized(Org. Lett. 2009, 11, 5614.DOI: 10.1021/ol9023375) the diastereoselective Co-catalyzed oxidative cyclization of 13 to 14.
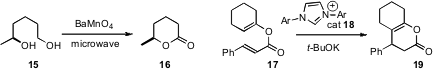
Mark C. Bagley and Andrew E. Graham of Cardiff University found(Tetrahedron Lett. 2009, 50, 6823.DOI: 10.1016/j.tetlet.2009.09.117)that microwave heating promoted the selective BaMnO4 oxidation of1,3-, 1,4-, and 1,5-diols such as 15 to the corresponding lactones.David W. Lupton of Monash University developed(J. Am. Chem. Soc. 2009, 131, 14176.DOI: 10.1021/ja905501z)the remarkable cyclization of 17 to 19, catalyzed by the carbeneprecursor 18.
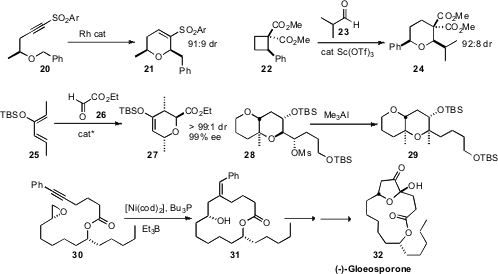
Hirokazu Urabe of the Tokyo Institute of Technology showed(J. Am. Chem. Soc. 2009, 131, 3166.DOI: 10.1021/ja809826a)that the unusual Rh-catalyzed cyclization of an alkynyl sulfone 20 proceededwith substantial diastereocontrol, delivering the cyclic ether 21 as the majorproduct. Jeffrey S. Johnson of the University of North Carolina established(J. Am. Chem. Soc. 2009, 131, 14202.DOI: 10.1021/ja906755e)that Sc(OTf)3 was a particularly efficient catalyst for theopening of 22 with 23 and then reclosure, leading to 24.Steven D. R. Christie and Gareth J. Pritchard of Loughborough University(Chem. Commun. 2009, 7339.DOI: 10.1039/B917332C)and Michael A. Kerr, also of the University of Western Ontario(J. Org. Chem. 2009, 74, 8414.DOI: 10.1021/jo9019122)investigated related condensations. Masahiro Terada of Tohoku University observed(J. Am. Chem. Soc. 2009, 131, 12882.DOI: 10.1021/ja904749x)that a diaryl binol phosphoric ester promoted the condensation of 25 with 26 to give the anti adduct 27 in high ee. With Lewis acid catalysts, the all-cis diastereomer predominated. The establishment of quaternary centers on cyclic ethers is particularly challenging. Tadashi Nakata of the Tokyo University of Science demonstrated(Org. Lett. 2009, 11, 4814.DOI: 10.1021/ol9018419)that ionization of 28 was followed by hydride transfer. Methylation of theresulting tertiary carbocation proceeded with high diastereocontrol, to give 29.
In the course of a synthesis of the macrolide (-)-Gloeosporone (32), Timothy F. Jamison of MIT demonstrated(Angew. Chem. Int. Ed. 2009, 48, 5366.DOI: 10.1002/anie.200902079)that the cyclization of the easily-assembled epoxy alkyne 30 to 31 proceeded smoothly with stoichiometric Ni. The reaction was best run 0.075 M in 30, an unusually high concentration for a macrocyclization.