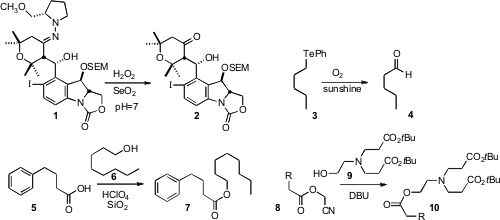
Amos B. Smith III of the University of Pennsylvania found
(Synlett 2009, 3131.
DOI: 10.1055/s-0029-1218352)
that the advanced SAMP intermediate 1 could be
deprotected to 2 without racemization under mild oxidative conditions. 6-Bromo-2,7-naphthyridin-1(2H)-one web
Akihiko Ouchi of the National Institute of Advanced Industrial Science and
Technology, Tsukuba, showed
(Org. (S)-(Tetrahydrofuran-3-yl)methanol web Lett. 2009, 11, 4870.
DOI: 10.1021/ol901943f)
that the C-Te of 3 was easily oxidized to the aldehyde 4.
Secondary C-Te bonds were converted to ketones. Asit K. Chakraborti of NIPER prepared
(J. Org. Chem. PMID:25955218 2009, 74, 5967.
DOI: 10.1021/jo900614s)
esters by warming an acid 5 with an alcohol 6 in the presence of acidic
silica gel. Gilles Quéléver of Aix-Marseille Université established
(Tetrahedron Lett. 2009, 50, 4346.
DOI: 10.1016/j.tetlet.2009.05.034)
that a cyanomethyl ester 8, readily prepared from the acid, efficiently
exchanged with an alcohol 9 to give the ester 10.
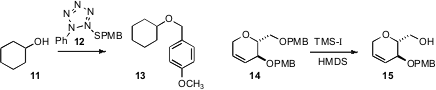
Martin J. Lear of the National University of Singapore protected
(Tetrahedron Lett. 2009, 50, 5267.
DOI: 10.1016/j.tetlet.2009.07.020)
an alcohol 11 as the p-methoxybenzyl ether 13
under mild conditions (AgOTf/DTBMP) with with the new reagent 12.
Isao Kadota of Okayama University selectively
(Tetrahedron Lett. 2009, 50, 4552.
DOI: 10.1016/j.tetlet.2009.05.084)
removed the primary PMB ether from the 14 to give 15.
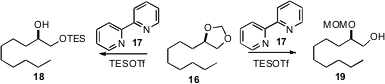
Hiromishi Fujioka of Osaka University, starting
(Org. Lett. 2009, 11, 5138.
DOI: 10.1021/ol902088b)
from 16, was able to selectively prepare either the
primary protected 18 or the secondary protected 19. In other
developments, not pictured, Mattie S. M. Timmer and Brendan A. Burkett of the
Victoria University of Wellington devised
(Tetrahedron Lett. 2009, 50, 7199.
DOI: 10.1016/j.tetlet.2009.10.043)
a convenient preparation for azulene-containing α-keto esters.
The distinctively-colored protecting group was conveniently removed in the
presence of other esters by treatment with o-phenylenediamine. Scott D. Taylor
of the University of Waterloo established
(J. Org. Chem. 2009, 74, 9406.
DOI: 10.1021/jo901882f)
a robust protocol for converting alcohols to the corresponding protected sulfates.
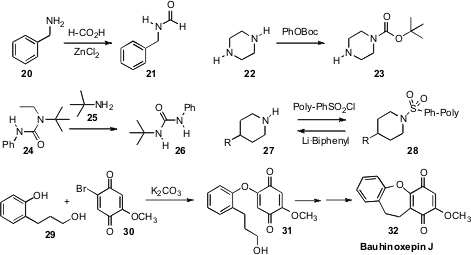
P. Shanthan Rao of the Indian Institute of Chemical Technology, Hyderabad, showed
(Tetrahedron Lett. 2009, 50, 7099.
DOI: 10.1016/j.tetlet.2009.10.006)
that an amine 20 was formylated by warming with formic acid in the presence of
ZnCl2. The easily-hydrolyzed formamide 21 is readily converted to
the corresponding isonitrile. Shiyue Fang of Michigan Technological University selectively
(Tetrahedron Lett. 2009, 50, 5741.
DOI: 10.1016/j.tetlet.2009.07.142)
monoacylated the symmetrical diamine 22 using phenyl esters. Guy C.
Lloyd-Jones and Kevin I. Booker-Milburn of the University of Bristol observed
(Angew. Chem. Int. Ed. 2009, 48, 8721.
DOI: 10.1002/anie.200904435)
that on gentle warming, the congested urea 24 released phenyl isocyanate. Rolf Breinbauer
of the Graz University of Technology established
(Angew. Chem. Int. Ed. 2009, 48, 5841.
DOI: 10.1002/anie.200901643)
that polystyrene sulfonyl chloride is a robust and easily removable protecting group.
George A. Kraus of Iowa State University showed
(Tetrahedron Lett. 2009, 50, 5303.
DOI: 10.1016/j.tetlet.2009.06.143)
that the phenol of 29 could be selectively protected by reaction with the
bromoquinone 30. Although the protecting group could be removed, in this case
the coupled product 31 was carried on to the antimalarial dibenz[bf]oxepin
Bauhinoxepin J (32).