(-)-Adaline (Yu), (-)-Securinine (Bayón/Figueredo), Alkaloid 223A (Aubé), (-)-Huperzine
A (Fukuyama)
The recent development of practical methods for the asymmetric preparation of
amines has enabled creative approaches to alkaloid construction. Cyclopropylmethyl bromide web Günter Helmchen
of the Ruprecht-Karls-Universität Heidelberg developed
(Synlett 2009, 1413.
DOI: 10.1055/s-0029-1217165)
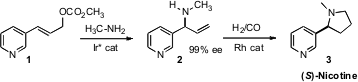
an iridium catalyst that mediated the enantioselective amination of the allylic
carbonate 1 to give 2. PMID:24761411 Hydroformylation followed by reduction then completed the
synthesis of (S)-nicotine (3).
Yian Shi of Colorado State University devised
(J. Org. Chem. 2009, 74, 7577.
DOI: 10.1021/jo9015584)
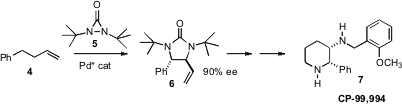
a Pd catalyst for the enantioselective oxidative diamination of terminal alkenes
such as 4. Price of 5-Bromo-1,3-thiazole-2-carbaldehyde The product 6 was readily carried on to (+)-CP-99,994, a potent and
selective nonpeptide substance P receptor antagonist.
Chan-Mo Yu of Sungkyunkwan University prepared
(Synlett 2009, 1498.
DOI: 10.1055/s-0029-1217172)
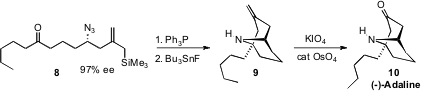
the alcohol corresponding to the azide 8 by BINOL-catalyzed addition of an allyl
stannane to the corresponding aldehyde. Reduction of the azide and subsequent
intramolecular condensation with the ketone gave an imine, that was cyclized
with Bu3SnF to 9. Oxidative cleavage then delivered (-)-Adaline
(10).
Barry M. Trost of Stanford University developed a family of Pd catalysts for the
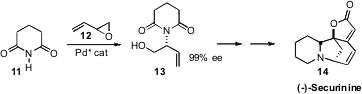
enantioselective coupling of racemic butadiene monoepoxide 12 with a range of
nucleophiles. Pau Bayón and Marta Figueredo of the Universitat Autónoma de Barcelona extended
(J. Org. Chem. 2009, 74, 6199.
DOI: 10.1021/jo901059n)
that range to include glutarimide 11 and succinimide. The adduct 13 provided the
enantiomerically-pure core for a total synthesis of (-)-Securinine (14), and others of
the Securinega alkaloids.
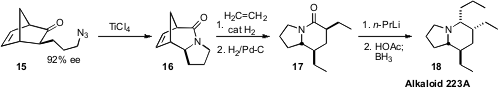
Jeffrey Aubé of the University of Kansas prepared
(Org. Lett. 2009, 11, 4140.
DOI: 10.1021/ol901645j)
the enantiomerically-enriched ketone 15 by the enantioselective
hydroboration of norbornadiene, followed by oxidation and alkylation.
Intramolecular Schmidt cyclization following the protocol he had developed
converted 15 into 16. He then took advantage of the still-substantial ring
strain of the expanded norbornene to drive ring-opening metathesis, to give,
after hydrogenation, the lactam 17. He was able to selectively convert 17 into
either 18 or the diastereomer in which the ethyl groups are cis one to another,
by varying the acid used in the final reductive work-up.
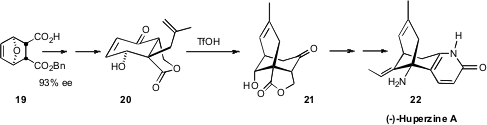
To prepare the bridged bicyclic core of (-)-Huperzine A (22), Tohru Fukuyama of
the University of Tokyo began
(Org. Lett. 2009, 11, 5354.
DOI: 10.1021/ol9022408)
with the monoester 19, readily prepared from the furan/maleic anhydride
Diels-Alder adduct by the
known quinine-mediated opening with benzyl alcohol. The aminated quaternary
center of 22 was established by inversion of the carboxy group of 21.