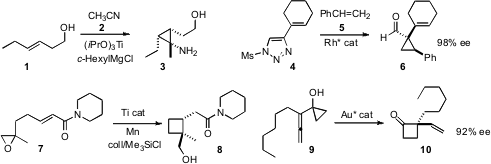
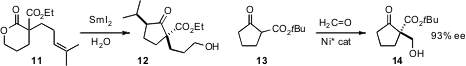Jin K. Cha of Wayne State University described
(J. Org. Chem. 2009, 74, 5528.
DOI: 10.1021/jo900823h)
the diastereoselective intramolecular
cyclopropanation of
nitriles with homoallylic alcohols such as 1. Valery V. Fokin of Scripps/LaJolla found
(J. Am. Chem. Soc. 2009, 131, 18034.
DOI: 10.1021/ja908075u)
that the diazoimine derived from 4 could add with high enantioselectivity to aryl
alkenes, including styrene 5.
Andreas Gansäuer of the University of Bonn optimized
(Angew. Chem. Int. 2-Isopropyl-6-nitroaniline web Ed. (R)-2-Amino-2-(3-bromophenyl)acetic acid Price 2009, 48, 8882,
DOI: 10.1002/anie.200904428;
Tetrahedron 2009, 65, 10791,
DOI: 10.1016/j.tet.2009.09.033)
a Ti catalyst for the cyclization of substrates such as 7 to the corresponding
cyclobutanes. PMID:35116795 F. Dean Toste of the University of California, Berkeley devised
(J. Am. Chem. Soc. 2009, 131, 9178.
DOI: 10.1021/ja904055z)
a gold catalyst for the enantioselective
ring expansion of a prochiral allene such as
9 to the cyclobutanone 10.
David J. Procter of the University of Manchester developed
(J. Am. Chem. Soc. 2009, 131, 15467.
DOI: 10.1021/ja906396u)
the SmI2-mediated cyclization of a lactone such as 11 to the cyclopentanone 12.
Shigeki Matsunaga and Masakatsu Shibasaki of the University of Tokyo designed
(Chem. Commun. 2009, 5138.
DOI: 10.1039/B912380F)
a Ni catalyst for the enantioselective condensation of 13 with formaldehyde.
Some acyclic β-keto esters could also be hydroxymethylated with high enantiocontrol.
Darren J. Dixon, of the University of Oxford, devised
(J. Am. Chem. Soc. 2009, 131, 9140.
DOI: 10.1021/ja9004859)
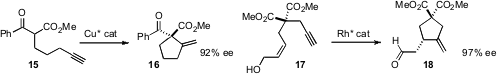
a Cu catalyst for the enantioselective
Conia cyclization of
15 to 16. K. C. Nicolaou, also of Scripps/La Jolla, reported
(Angew. Chem. Int. Ed. 2009, 48, 6293.
DOI: 10.1002/anie.200901992)
a Rh catalyst for the related cyclization of 17 to 18.
Ryo Shintani and Tamio Hayashi of Kyoto University showed
(J. Am. Chem. Soc. 2009, 131, 13588.
DOI: 10.1021/ja905432x)
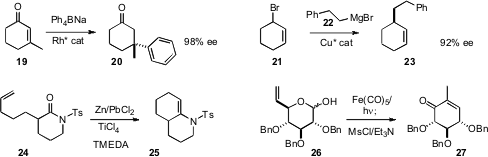
that a Rh catalyst could effect enantioselective conjugate addition of a tetraaryl borate even
to a 3-substituted cyclohexenone 19, to establish the cyclic quaternary center.
Alexandre Alexakis of the University of Geneva established
(Chem. Commun. 2009, 3868.
DOI: 10.1039/B907722G)
that with an easily ionized racemic allylic bromide 21, Cu-mediated coupling
with the alkyl Grignard 22 proceeded with substantial asymmetric induction.
Jon D. Rainier of the University of Utah devised
(Org. Lett. 2009, 11, 3774.
DOI: 10.1021/ol901448n)
conditions for effecting Ti-mediated intramolecular metathesis between an alkene and a lactam
carbonyl, to give the cyclic enamide 24. Jhillu Singh Yadav of the Indian Institute of
Chemical Technology, Hyderabad and René Grée of the Université de Rennes 1 effected
(Chem. Commun. 2009, 4717.
DOI: 10.1039/B907632H)
isomerization of the alkenyl ether 26 to the ethyl ketone, leading, via intramolecular
aldol condensation and dehydration, to the enone 27.
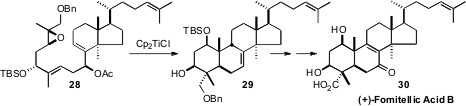
In the course of a total synthesis of the DNA polymerase inhibitor (+)-Fometillic
Acid B (30), Susumu Kobayashi of Tokyo University found
(Tetrahedron Lett. 2009, 50, 6764.
DOI: 10.1016/j.tetlet.2009.09.088)
that the Ti (III) reagent developed by W. A. Nugent and T. V RajanBabu effected cyclization of
28 to 29. The alternative diastereomer of the secondary acetate did not cyclize efficiently.