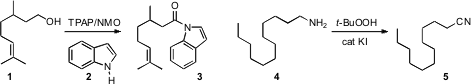
Karl A. 6-Bromo-7-fluoroisobenzofuran-1(3H)-one uses Scheidt of Northwestern University described(Org. Lett. PMID:34645436 2009, 11, 1651.DOI: 10.1021/ol900306v)the oxidation of primary alcohols such as 1 in the presence of an indole 2. The product 3, an active acylating agent, is readily converted to other esters and amides. K. Rajender Reddy of the Indian Institute of Chemical Technology, Hyderabad developed(Tetrahedron Lett. 2009, 50, 2050.DOI: 10.1016/j.tetlet.2009.02.057)a protocol for the direct oxidation of a primary amine4 to the corresponding nitrile 5. In the presence of ammonia, the same procedure converted aldehydes and primary alcohols into the nitriles. 2-Bromo-6-iodoaniline supplier
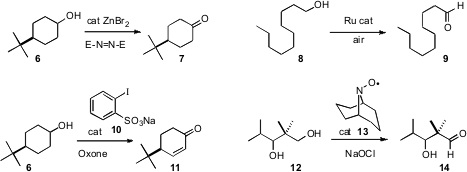
Several catalytic methods for the oxidation of alcohols to aldehydes and ketoneshave recently been put forward. René Grée of the Université de Rennes 1 found(Tetrahedron Lett. 2009, 50, 1493.DOI: 10.1016/j.tetlet.2009.01.080)that ZnBr2 catalyzed the oxidation of alcohols withdiethylazodicarboxylate. Tsutomu Katsuki of Kyushu University designed(Tetrahedron Lett. 2009, 50, 3432.DOI: 10.1016/j.tetlet.2009.02.169)a Ru catalyst for the air oxidation of primary alcohols to aldehydes. Kazuaki Ishihara of Nagoya University showed(J. Am. Chem. Soc. 2009, 131, 251.DOI: 10.1021/ja807110n)that 1 mol % of 10 was sufficient to catalyze the oxidation of 6 to 7. With excess oxidant, 7 was carried on cleanly to 11.
Nitroxyl radicals such as TEMPO have long been used tocatalyze oxidations. Yoshiharu Iwabuchi of Tohoku University developed(J. Org. Chem. 2009, 74, 4619.DOI: 10.1021/jo900486w)a simple preparation of 13, the most efficient such catalyst so far reported. This catalyst should also be useful for the oxidation reported by Professor Iwabuchi(Chem. Commun. 2009, 1739.DOI: 10.1039/b822944a)of primary alcohols and aldehydes to the corresponding carboxylic acids.
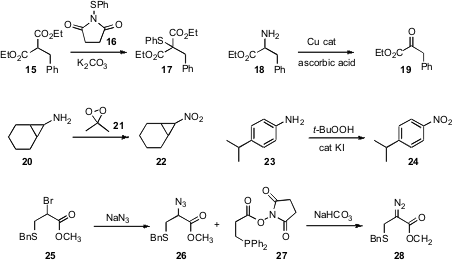
David S. Forbes of the University of South Alabama prepared(Tetrahedron Lett. 2009, 50, 1855.DOI: 10.1016/j.tetlet.2009.02.169)16 by combining thioanisole withN-bromosuccinimide. The reagent 16efficiently sulfenylated active methylene compounds. Jiri Srogl of the Academyof Sciences of the Czech Republic established(Org. Lett. 2009, 11, 843.DOI: 10.1021/ol802715c)conditions for the oxidation of primary and secondary amines to aldehydes andketones. Olga A. Ivanova of Moscow State University demonstrated(Tetrahedron Lett. 2009, 50, 2793.DOI: 10.1016/j.tetlet.2009.03.165)that DMDO (21) could oxidize a sensitive amino cyclopropane suchas 20 to the corresponding nitro compound. Professor Reddy found(Adv. Synth. Catal. 2009, 351, 93.DOI: 10.1002/adsc.200800641)that conditions similar to those that converted 4 to 5 also oxidized 23 to 24.
Ronald T. Raines of the University of Wisconsin devised(Angew. Chem. Int. Ed. 2009, 48, 2359.DOI: 10.1002/anie.200804689)the reagent 27 to convert an organic azide such as 26 to the corresponding diazo compound 27. A wide variety of diazo compounds, including some that it would be difficult to synthesize by other approaches, were prepared using this protocol.