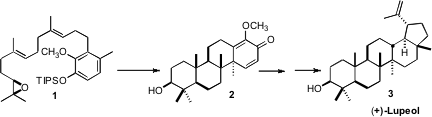
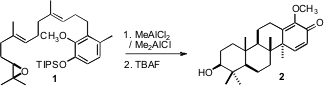The total synthesis of lupeol was one of the crowning achievements of the
Robinson annulation/reductive alkylation approach to stereocontrolled
polycarbocyclic construction developed by Gilbert Stork
(J. Am. Chem. Soc. 1971, 93, 4945.
DOI: 10.1021/ja00748a068).
It is a measure of the progress of organic synthesis since
that time that E. J. Dde-Dap(Fmoc)-OH site Corey of Harvard University could devise
(J. Am. Chem. PMID:24487575 5-Chloro-2,3-dimethylpyrazine Chemscene Soc. 2009, 131, 13928.
DOI: 10.1021/ja906335u)
an enantioselective synthesis of (+)-lupeol (3) that could be carried
out by a single co-worker. The key step in the synthesis was the Lewis
acid-mediated cyclization of 1 to 2.
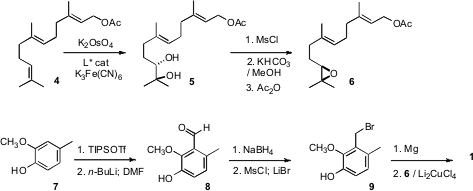
The preparation of 1 began with the enantioselective epoxidation of
farnesol acetate (4). To this end, asymmetric
dihydroxylation delivered
the diol 5. Selective mesylation followed by exposure to dilute methoxide
effected ring closure to the epoxide, but also removed the acetate, so this had
to be reapplied.
The synthesis of the aromatic portion of 1 started with the phenol
7. Protection as the very bulky triisopropylsilyl ether was important for
the success of the subsequent cyclization, perhaps because it discouraged
complexation of the Lewis acid with the aryl ether. Metalation followed by
formylation delivered the aldehyde 8, that was reduced and carried on to
the bromide 9. The derived
Grignard reagent coupled smoothly with 6
under Li2CuCl4 catalysis.
The cyclization of 1 to 2 proceeded with remarkable efficiency
(43%!), for a reaction in which three new carbon-carbon bonds, four rings and
five new stereogenic centers were established. It is particularly noteworthy
that the cyclization cleanly set the trans, anti, trans, anti tetracyclic
backbone of (+)-lupeol (3).
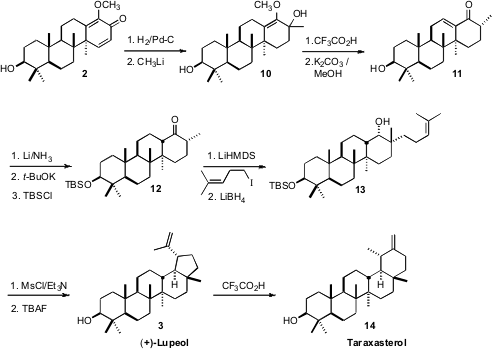
To complete the synthesis of 3, the less substituted alkene of 2
was selectively hydrogenated, then CH3Li was added to give 10.
Hydrolysis and dehydration yielded 11, that was reduced and equilibrated
to 12. On brief exposure to MsCl/Et3N, 13 cyclized to
(+)-lupeol (3).
It is a measure of the remarkable efficiency of this synthesis of (+)-lupeol
(3) that it provided sufficient material to enable studies of the
rearrangement of 3 under acidic conditions to other pentacyclic
triterpenes, including, inter alia, germanicol, α-amyrin, β-amyrin and
taraxasterol (14).