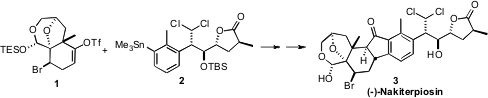
(-)-Nakiterpiosin (3), isolated from the thin encrusting sponge
Terpios hoshinota, has an IC50 against murine P388 leukemia cells
of 10 ng/mL. Chuo Chen of UT Southwestern Medical Center developed
(J. Am. PMID:28322188 Chem. Soc. Formula of 2377610-54-1 2010, 132, 371.
DOI: 10.1021/ja908626k)
a practical synthetic route to 3 based on the convergent coupling of 1 and 2.
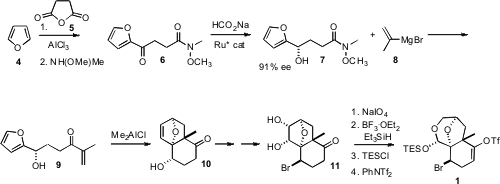
The preparation of 1 was based on the intramolecular [4+2] cyclization
of the furan 9, prepared by
Friedel-Crafts acylation of furan (4)
with maleic anhydride (5). The absolute configuration of the secondary
alcohol was set by Noyori reduction, using
sodium formate as the hydride source. N2-Isobutyryl-2′-O-methylguanosine web
The cyclization of 9 to 10 proceeded with high diastereocontrol,
presumably by way of a chelated transition state. As expected, cyclization of
the silyl ether of 9 delivered the complementary diastereomer. As the
cyclization of 9 was readily reversible, it was taken quickly to the
bromide 11. Oxidative cleavage of the diol followed by selective
reduction and protection then completed the synthesis of 1.
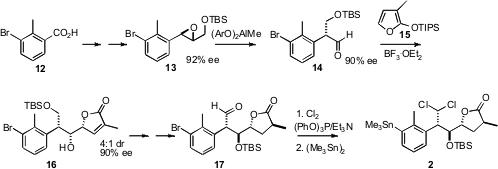
The preparation of 2 began with the commercial bromo acid 12.
The enantiomerically-enriched epoxide 13 was constructed in the usual
way, by homologation of the aldehyde to the allylic alcohol followed by
Sharpless epoxidation. On exposure to the Yamamoto catalyst, 13 smoothly
rearranged to the aldehyde 14. Condensation of 14 with 15
then gave 16, with only minimal erosion of enantiomeric excess over the
two steps.
Unfortunately, 16 was the incorrect diastereomer, so it had to be
inverted. With the aldehyde 17 in hand, conversion to the dichloride
followed by functional group interchange completed the construction of 2.
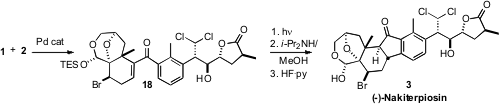
Carbonylative coupling of 1 and 2 led to the enone 18.
The photochemical Nazarov cyclization of 18 proceeded with the expected
high diastereocontrol, to give, after epimerization, the desired
trans-anti-trans product. Deprotection then completed the synthesis of (-)-Nakiterpiosin
(3). It is noteworthy that the full A-ring functionality of 3 was
compatible with the conditions of the photochemical cyclization.
The work of Chen toward the total synthesis of (-)-nakiterpoisin (3) led to a
correction of the relative configurations both of the dichloromethyl substituent
and of the secondary bromide. The availability of 3 by total synthesis is
particularly exciting, because it has been shown to interfere with the Hedgehog
signaling pathway. There is the potential, based on this activity, that
derivatives of 3 may prove useful as adjuncts in cancer chemotherapy.