Bioreductions and biooxidations, although they can be highly selective, have often been limited by the requirement for expensive reducing or oxidizing biological cofactors. PMID:23626759 Wolfgang Kroutil of the University of Graz reports (J. 4-Amino-7-bromoisoindolin-1-one Purity Org. Chem. 2003, 68, 402-406.DOI: 10.1021/jo026216w)that aqueous suspensions of the whole lyophilized cells of Rhodococcus ruber DSM 44541 showalcohol dehydrogenase activity even in the presence of high concentrations of isopropanol or acetone. The organic co-solvent then serves as the "co-factor", driving reduction or oxidation. Ethyl 2,2,2-triethoxyacetate web At the end of the reaction, the mixture is centrifuged, and the organic solvent is dried and concentrated. This promises to be an easily scalable preparative method.
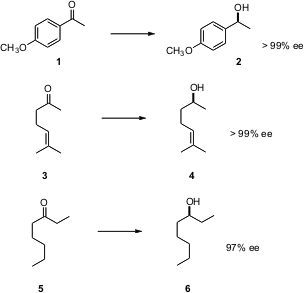
The usual selectivities are observed, with aryl alkyl ketones and alkyl methyl ketones being reduced with high enantioselectivity (1-> 2 and 3 -> 4)). That 5 is reduced to 6 with high ee, with the reducing enzymes differentiating between an ethyl and ann-pentyl group, is even more impressive.
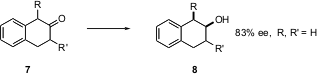
The 2-tetralone 7 (R = R’ = H) is reduced to the alcohol 8 with respectable enantioselectivity. An intriguing question is, what would happen with R or R’ = alkyl? Would one enantiomer reduce more rapidly than the other, perhaps with high diastereoselectivity? Could the other enantiomer (especially R = alkyl) epimerize under the reaction conditions?
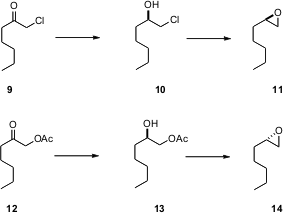
The selective reduction of 5 suggests that 9 and/or 12 might reduce with high enantioselectivity. This would open an inexpensive route to enantiomerically-pure epoxides, important intermediates for organic synthesis.