A
Tanmaya Pathak of the Indian Institute of Technology, Kharagpur devised
(J. Org. Price of 6-Chloro-3-fluoro-2-methoxypyridine Chem. NH2-PEG2-C2-Boc structure 2009, 74, 2710.
DOI: 10.1021/jo802709q)
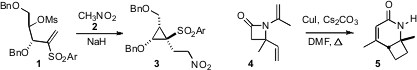
a preparation of enantiomerically-pure oxygenated
cyclopropanes such as 3 from
carbohydrate precursors. Andrei K. Yudin of the University of Toronto established
(Org. Lett. 2009, 11, 1281.
DOI: 10.1021/ol900118d)
a route to aminated
cyclobutanes such as 5 based on sigmatropic
rearrangement of the β-lactam 4. PMID:24140575
Stephen C. Bergmeier of Ohio University reported
(Tetrahedron 2009, 65, 741.
DOI: 10.1016/j.tet.2008.11.043)
a study of the balance between five- and six-membered ring formation in the
cyclization of aziridines such as 6. Professor Bergmeier also described
(Tetrahedron Lett. 2009, 50, 1261.
DOI: 10.1016/j.tetlet.2008.12.098)
the bridging additions of enones to cyclic allyl silanes such as 8.
This is particularly interesting, as 8 is easily prepared by
Birch
reduction of the corresponding phenyl silane.
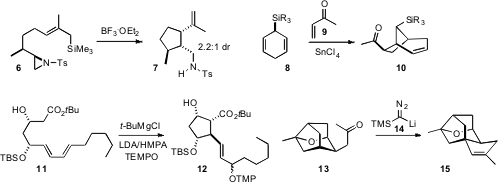
Ullrich Jahn of the Academy of Sciences of the Czech Republic observed
(Chem. Eur. J. 2009, 15, 58.
DOI: 10.1002/chem.200802139)
that the free-radical cyclization of 11 proceeded to give
mainly the diastereomer 12 (~ 1:1 at the secondary allylic position). Daesung
Lee of the University of Illinois at Chicago reasoned
(J. Am. Chem. Soc. 2009, 131, 8413.
DOI: 10.1021/ja903526g)
that the stereochemical relationship between the O and the adjacent
C-H of 13 was such that the C-H would be deactivated. The cyclization of the
alkylidene carbene derived from 13 indeed proceeded to give 14, setting the
stage for the synthesis of platensimycin.
Marco A. Cufolini of the University of British Columbia found
(Org. Lett. 2009, 11, 1539.
DOI: 10.1021/ol900194v)
an easy protocol for the generation of a nitrile oxide and subsequent
dipolar cycloaddition, by oxidation of the oxime. In a related investigation,
Adam J. M. Burrell and Iain Coldham of the University of Sheffield cyclized
(Org. Lett. 2009, 11, 1515.
DOI: 10.1021/ol9001653)
the oxime derived from 18, by way of the intermediate nitrone, to give
19 with high diastereocontrol.
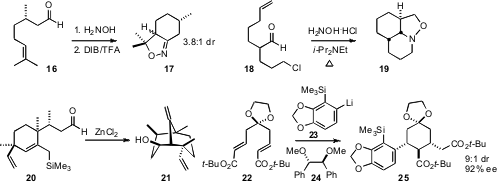
Toshio Honda of Hoshi University established
(J. Org. Chem. 2009, 74, 3424.
DOI: 10.1021/jo900369t)
that the intramolecular
Sakurai cyclization of 20 proceeded with high
diastereocontrol, to give 21. Kiyoshi Tomioka of Kyoto University showed
(Org. Lett. 2009, 11, 1631.
DOI: 10.1021/ol9003564)
that the chiral ligand 24 directed the absolute course of the cascade
addition of 23 to 22. The product 25 was carried on to (-)-Lycorine.
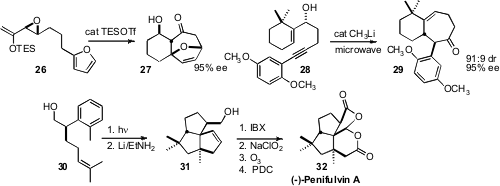
Pauline Chiu of the University of Hong Kong devised
(J. Am. Chem. Soc. 2009, 131, 4556.
DOI: 10.1021/ja807566t)
conditions for the cyclization of the epoxide 26 to 27, with the
enantiomerically-pure epoxide controlling the absolute configuration of the
tricyclic ring system. Timo V. Ovaska of Connecticut College set
(Org. Lett. 2009, 11, 2715.
DOI: 10.1021/ol900967j)
the absolute configuration of 28 by
Itsuno-Corey reduction of
the corresponding ketone. Cascade cyclization then delivered 29. Tanja Gaich and
Johann Mulzer of the University of Vienna designed
(J. Am. Chem. Soc. 2009, 131, 452.
DOI: 10.1021/ja8083048)
a short route to the dioxafenestrane insecticide (-)-Pentifulvin A (32).
Photocyclization converted the alcohol 30 to a ~ 1:1 mixture of regioisomers.
Reduction of one of them gave 31, which was oxidized to 32.