Several new methods for the direct functionalization of Ar-H have appeared. PMID:24377291
Hisao Yoshida of Nagoya University observed
(Chem. Comm. 2008, 4634.
DOI: 10.1039/b811555a)
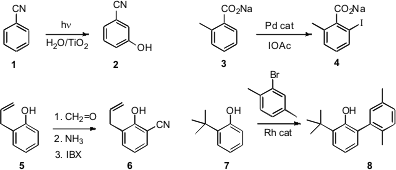
that under irradiation, TiO2 in water effected meta
hydroxylation of benzonitrile 1 to give the phenol 2. 6-Bromo-7-azaindole custom synthesis Anisole
showed ortho selectivity, while halo and alkyl aromatics gave mixtures.
Melanie S. Sanford of the University of Michigan reported
(J. Am. 5632-70-2 Chemscene Chem. Soc. 2008, 130, 13285.
DOI: 10.1021/ja8045519)
a complementary study of Pd-catalyzed ortho
acetoxylation. Jin-Quan Yu of Scripps/La Jolla developed
(Angew. Chem. Int. Ed. 2008, 47, 5215.
DOI: 10.1002/anie.200705613)
a Pd-catalyzed protocol for ortho
halogenation of aromatic carboxylates such as 3. A related protocol
(J. Am. Chem. Soc. 2008, 130, 17676.
DOI: 10.1021/ja806681z)
led to ortho arylation. Trond Vidar Hansen of the University of Oslo devised
(Tetrahedron Lett. 2008, 49, 4443.
DOI: 10.1016/j.tetlet.2008.05.006)
a one-pot procedure for the net ortho
cyanation of phenols such as 5 to the salicylnitrile 6. Robin B.
Bedford of the University of Bristol, Andrew J. M. Caffyn of the University of
the West Indies and Sanjiv Prashar of the Universidad Rey Juan Carlos established
(Chem. Comm. 2008, 990.
DOI: 10.1039/b718128k)
a Rh-catalyzed protocol for ortho arylation of phenols such as 7.
Laurent Désaubry of the Université Louis Pasteur observed
(Tetrahedron Lett. 2008, 49, 4588.
DOI: 10.1016/j.tetlet.2008.05.091)
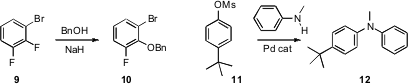
regioselective coupling of unsymmetrical
difluorobenzenes such as 9 to give the ether 10. Fuk Yee Kwong of
Hong Kong Polytechnic University extended
(Angew. Chem. Int. Ed. 2008, 47, 6402.
DOI: 10.1002/anie.200802157)
Pd-mediated amination to the notoriously difficult mesylates,
such as 11. John F. Hartwig of the University of Illinois reported
(J. Am. Chem. Soc. 2008, 130, 13848
DOI: 10.1021/ja805810p)
a related method for the amination of aryl tosylates.
Hong Liu of the Shanghai Institute of Materia Medica found
(Org. Lett. 2008, 10, 4513.
DOI: 10.1021/ol801784a)
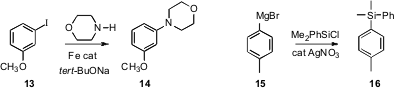
that the Fe-catalyzed amination of aryl halides
such as 13 sometimes gave mixtures of regioisomers. Hideki Yorimitsu and
Koichiro Oshima of Kyoto University effected
(Angew. Chem. Int. Ed. 2008, 47, 5833.
DOI: 10.1002/anie.200801949)
Ag-catalyzed Grignard cross coupling with aryl
halides, converting 15 into 16. Note that silyl aromatics such as
16 are readily reduced under dissolving metal conditions to give allyl
silanes.
Frederic G. Buono of the Bristol-Myers Squibb chemical process group optimized
(Org. Lett. 2008, 10, 5325.
DOI: 10.1021/ol8016935)
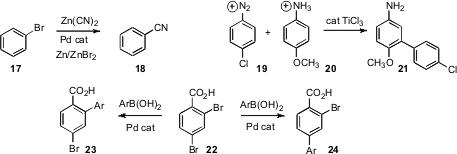
the cyanation of an aryl halide
17, uncovering the valuable co-catalytic role of ZnBr2.
Markus R. Heinrich of the Technische Universität München developed
(Angew. Chem. Int. Ed. 2008, 47, 9130.
DOI: 10.1002/anie.200803785)
an intriguing Ti(III)-catalyzed radical arylation, converting 20 into
21.
Ioannis N. Houpis of the Johnson and Johnson chemical process group established
(Org. Lett. 2008, 10, 5601.
DOI: 10.1021/ol802349u)
conditions for Pd coupling to selectively convert 22 to either 23 or 24.
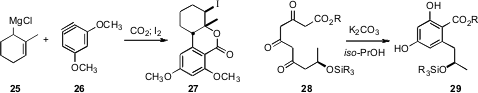
Anthony G. M. Barrett of Imperial College London took advantage
(Org. Lett. 2008, 10, 3833.
DOI: 10.1021/ol8015435)
of regioselective addition the allylic anion 25
to the benzyne 26 to give, after carboxylation, the iodolactone 27,
that he carried on to Dehydroaltenuene B. Following up on the work of Thomas M.
Harris, Professor Barrett also demonstrated
(J. Am. Chem. Soc. 2008, 130, 10293.
DOI: 10.1021/ja803445u)
the facile aromatization of the triketone 28 to the
arene 29, that he carried on to the antifungal agent 15G256β.