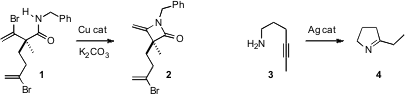
Chaozhong Li of the Shanghai Institute of Organic Chemistry demonstrated
(Org. Lett. 2008, 10, 4037
DOI: 10.1021/ol801545a)
facile and selective Cu-catalyzed
β-lactam formation, converting 1 to 2.
Paul Helquist of the University of Notre Dame devised
(Org. Lett. 2008, 10, 3903
DOI: 10.1021/ol801458g)
an effective catalyst for intramolecular alkyne hydroamination, converting 3 into the
imine 4. Six-membered ring construction worked well also.
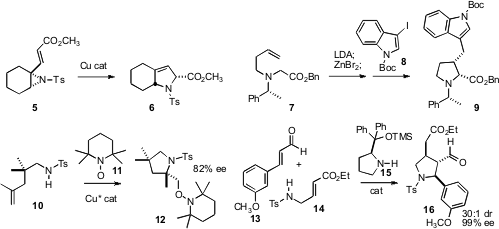
Jon T. 1783945-29-8 Purity (S)-BI-DIME uses Njardarson of Cornell University found
(Org. PMID:24220671 Lett. 2008, 10, 5023
DOI: 10.1021/ol802123e)
a Cu catalyst for the rearrangement of alkenyl aziridines such
as 5 to the pyrroline 6. Philippe Karoyan of the UPMC, Paris developed
(J. Org. Chem. 2008, 73, 6706
DOI: 10.1021/jo801006a)
an interesting chiral auxiliary directed cascade process,
converting the simple precursor 7 into the complex
pyrrolidine 9.
Sherry R. Chemler of the State University of New York, Buffalo devised
(J. Am. Chem. Soc. 2008, 130, 17638
DOI: 10.1021/ja806585m)
a chiral Cu catalyst for the cyclization of 10, to give 12
with substantial enantiocontrol. Wei Wang of the University of New Mexico demonstrated
(Chem. Commun. 2008, 5636
DOI: 10.1039/b812464g)
the organocatalyzed condensation of 13 and 14 to give 16
with high enantio- and diastereocontrol.
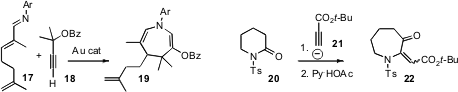
Two complementary routes to azepines/azepinones have appeared. F. Dean Toste
of the University of California, Berkeley showed
(J. Am. Chem. Soc. 2008, 130, 9244
DOI: 10.1021/ja803890t)
that a gold complex catalyzed the condensation of 17 and 18 to give
19. Frederick G. West of the University of Alberta found
(Org. Lett. 2008, 10, 3985
DOI: 10.1021/ol8014682)
that lactams such as 20 could be ring-expanded by the addition of the
propiolate anion 21.
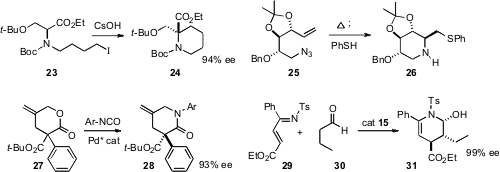
Takeo Kawabata of Kyoto University extended
(Org. Lett. 2008, 10, 3883
DOI: 10.1021/ol801213c)
“memory of chirality” studies to the cyclization of 23, demonstrating that
24 was formed in high ee. Paul V. Murphy of University College Dublin took advantage
(Org. Lett. 2008, 10, 3777
DOI: 10.1021/ol8014495)
of the well-known intramolecular
addition of azides to alkenes, showing that the intermediate could be
intercepted with nucleophiles such as thiophenol, to give the cyclized product
26 with high diastereocontrol. Ryo Shintani and Tamio Hayashi, also of Kyoto
University, found
(J. Am. Chem. Soc. 2008, 130, 16174
DOI: 10.1021/ja807662b)
that a chiral Pd catalyst effected condensation of racemic 27
with an aryl isocyanate to give, via decarboxylation, the lactam 28
in high ee. Ying-Chun Chen of Sichuan University optimized
(Angew. Chem. Int. Ed. 2008, 47, 9971
DOI: 10.1002/anie.200804183)
the organocatalyst-mediated condensation of 29 with 30 to give
31 with high enantio- and diastereocontrol.
Guillaume Bélanger of the Université de Sherbrooke devised
(Org. Lett. 2008, 10, 4939
DOI: 10.1021/ol802010n)
an elegant cascade dipole formation and cycloaddition,
converting 32 into 33 as a single diastereromer.