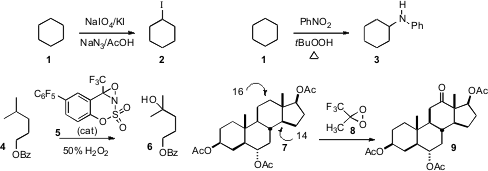
Arumugam Sudalai of the National Chemical Laboratory, Pune reported(Tetrahedron Lett. 2008, 49, 6401.DOI: 10.1016/j.tetlet.2008.08.071)a procedure for hydrocarbon iodination. With straight chain hydrocarbons, only secondary iodination was observed. 227454-58-2 site Chao-Jun Li of McGill University uncovered(Adv. Synth. Catal. 2009, 351, 353.DOI: 10.1002/adsc.200800689)a procedure for direct hydrocarbon amination, converting cyclohexane 1into the amine 3. Justin Du Bois of Stanford University established(Angew. Chem. Int. 4-(Vinylsulfonyl)benzoic acid uses Ed. 2009, 48, 4513.DOI: 10.1002/anie.200901353)a procedure for alkane hydroxylation, converting 4 selectively into the alcohol 6. PMID:24238415 The oxirane 8 usually also preferentially ozidizes methines, hydroxylating steroids at the C-14 position. Ruggero Curci of the University of Bari found(Tetrahedron Lett. 2008, 49, 5614.DOI: 10.1016/j.tetlet.2008.07.042)that the substrate 7 showed some C-14 hydroxylation, but also a useful yield of the ketone 9. The authors suggested that the C-7 acetoxy group may be deactivating the C-14 C-H.
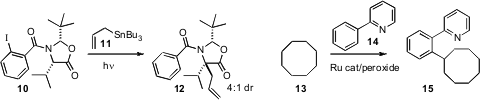
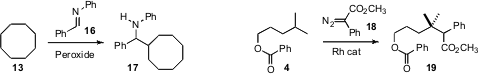
C-H bonds can also be converted directly to carbon-carbon bonds. Mark E. Wood of the University of Exeter found(Tetrahedron Lett. 2009, 50, 3400.DOI: 10.1016/j.tetlet.2009.02.110)that free-radical removal of iodine from 10 followed by intramolecular H-atom abstraction in the presence of the trapping agent11 delivered 12 with good diastereocontrol. Professor Li observed(Angew. Chem. Int. Ed. 2008, 47, 6278.DOI: 10.1002/anie.200801544)that under Ru catalysis, hydrocarbons such as 13 could be directly arylated. He also established(Tetrahedron Lett. 2008, 49, 5601.DOI: 10.1016/j.tetlet.2008.07.040)conditions for the direct aminoalkylation of hydrocarbons such as 13, to give 17. Huw M. L. Davies of Emory University converted(Synlett 2009, 151.DOI: 10.1055/s-0028-1087388)the ester 4 to the homologated diester 19 in preparatively useful yield using the diazo ester 18, the precursor to a selective, push-pull stabilized carbene.
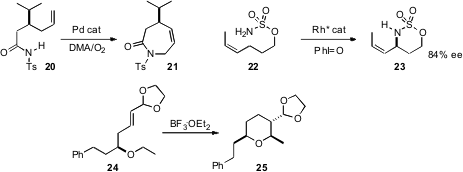
Intramolecular bond formation to an unactivated C-H can be even more selective. Guoshen Liu of the Shanghai Institute of Organic Chemistry developed (Org. Lett. 2009, 11, 2707.DOI: 10.1021/ol900941t)an oxidative Pd system that cyclized 20 to the seven-membered ring lactam 21.Professor Du Bois devised(J. Am. Chem. Soc. 2008, 130, 9220.DOI: 10.1021/ja8031955)a Rh catalyst that effected allylic amination of 22, to give 23with substantial enantiocontrol. Dalibor Sames of Columbia University designed(J. Am. Chem. Soc. 2009, 131, 402.DOI: 10.1021/ja806068h)a remarkable cascade approach to C-H functionalization. Exposure of 24to Lewis acid led to intramolecular hydride abstraction. Cyclization of the resultingstabilized carbocation delivered the tetrahydropyan 25 with remarkable diastereocontrol.
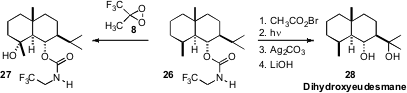
The oxidation(Nature 2009, 459, 824.DOI: 10.1038/nature08043)of the eudesmol carbamate 26 by Phil S. Baran of Scripps/La Jolla presents aninteresting case study. Direct intermolecular oxidation with the same reagent used by Professor Curci, TFDO (8), proceeded selectively, with retention of absolute configuration, to give the equatorial alcohol 27. In contrast, distal bromination directed by the carbamate followed by hydrolysis, using the protocol developed by the Baran group, gave the complementary diol, Dihydroxyeudesmane (28).