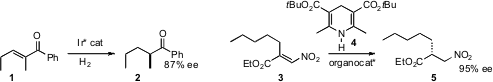
Carsten Bolm of RWTH Aachen developed
(Angew. Chem. Int. Ed. 2008, 47, 8920
DOI: 10.1002/anie.200803709)
an Ir catalyst that effected hydrogenation of trisubstituted enones such
as 1 with high ee. Benjamin List of the Max-Planck-Institut Mülheim devised
(J. Am. Chem. Soc. 2008, 130, 13862.
DOI: 10.1021/ja8069852)
an organocatalyst for the enantioselective reduction of nitro acrylates such as
3 with the Hantzsch ester 4.
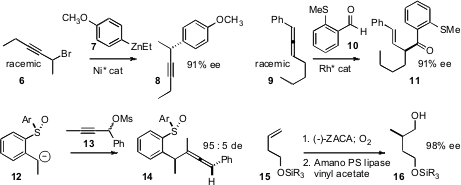
Gregory C. 3-Amino-4-methylpicolinic acid In stock Fu of MIT optimized
(J. PMID:23008002 Am. Chem. Soc. 2008, 130, 12645.
DOI: 10.1021/ja805165y)
a Ni catalyst for the enantioselective arylation of propargylic halides
such as 6. Both enantiomers of 6 were converted to the single
enantiomer of 8. Price of (R)-1-(2-Pyridyl)ethylamine Michael C. Willis of the University of Oxford established
(J. Am. Chem. Soc. 2008, 130, 17232.
DOI: 10.1021/ja8069133)
that hydroacylation with a Rh catalyst was selective for one enantiomer of the allene
9, delivering 11 in high ee. Similarly, José Luis García Ruano of
the Universidad Autónoma de Madrid found
(Angew. Chem. Int. Ed. 2008, 47, 6836.
DOI: 10.1002/anie.200802158)
that one enantiomer of racemic 13 reacted selectively
with the enantiomerically-pure anion 12, to give 14 in high
diastereomeric excess. Ei-ichi Negishi of Purdue University described
(Org. Lett. 2008, 10, 4311.
DOI: 10.1021/ol8017566)
the Zr-catalyzed asymmetric carboalumination (ZACA reaction) of the alkene 15
to give the useful chiron 16. Lipase was used to
polish the enantiomeric excess of 16.
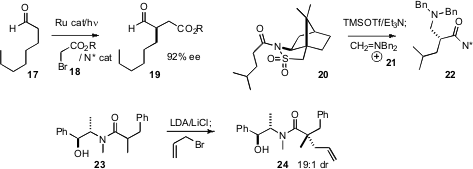
David W. C. MacMillan of Princeton University developed
(Science 2008, 322, 77.
DOI: 10.1126/science.1161976)
an intriguing visible light-powered
oxidation-reduction approach to enantioselective aldehyde alkylation. The
catalytic chiral secondary amine adds to the aldehyde to form an enamine, that
then couples with the radical produced by reduction of the haloester.
Two other alkylations were based on readily-available chiral
auxiliaries. Philippe Karoyan of the Université Pierre et Marie Curie observed
(Tetrahedron Lett. 2008, 49, 4704.
DOI: 10.1016/j.tetlet.2008.05.131)
that the acylated Oppolzer camphor sultam 20 condensed with the Mannich
reagent 21 to give 22 as a single diastereomer.
Andrew G. Myers of Harvard University developed the pseudoephedrine chiral auxiliary of
23 to direct the construction of ternary alkylated centers. He has now established
(J. Am. Chem. Soc. 2008, 130, 13231.
DOI: 10.1021/ja806021y)
that further alkylation gave 24, having a
quaternary alkylated center, in high diastereomeric excess.
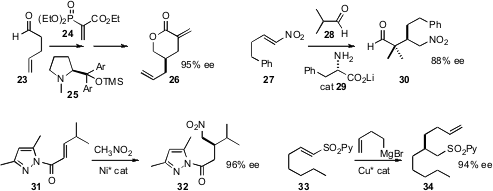
Karl Anker Jørgensen of Aarhus University developed
(J. Org. Chem. 2008, 73, 8337.
DOI: 10.1021/jo801582t)
an enantioselective route to 4-alkyl α-methylene
lactones
such as 26, based on conjugate addition of an aldehyde to the activated acceptor
24. Masanori Yoshida of Hokkaido University found
(Chem. Commun. 2008, 6242.
DOI: 10.1039/b814804j)
that the Li salt 29 of phenylalanine was an effective catalyst for the conjugate
addition of aldehydes to nitroalkenes such as 27. Masayuki Hasegawa and Shuji
Kanemasa of Kyushu University designed
(Tetrahedron Lett. 2008, 49, 5105,
DOI: 10.1016/j.tetlet.2008.05.153
5220, DOI: 10.1016/j.tetlet.2008.05.156)
a Ni catalyst for the enantioselective addition of nitromethane to acyl
pyrazoles such as 31. Ben L. Feringa of the University of Groningen established
(Org. Lett. 2008, 10, 4219.
DOI: 10.1021/ol801566n)
what may be one of the most flexible of approaches
to alkylated chirons, the enantioselective conjugate addition of Grignard
reagents to unsaturated sulfones such as 33.