(+)-6′-Hydroxyarenarol (Anderson)
V. T. Perchyonok and Kellie L. Tuck of Monash University found
(Tetrahedron Lett. 2008, 49, 4777.
DOI: 10.1016/j.tetlet.2008.05.097 )
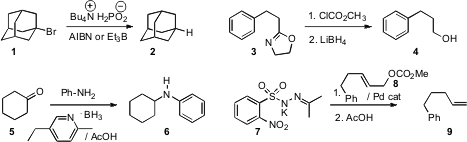
that a concentrated solution of Bu4NCl
and H3PO2 in water effected free radical reductions and
cyclizations. (S)-2-Methylpiperidine hydrochloride web Stéphane G. Ouellet of Merck Frosst demonstrated
(Tetrahedron Lett. 2008, 49, 6707.
DOI: 10.1016/j.tetlet.2008.09.061)
that an oxazoline such as 3 could be converted to the alcohol
4 by acylation followed by reduction. Elizabeth R. Burkhardt of BASF developed
(Tetrahedron Lett. PMID:23341580 2008, 49, 5152.
DOI: 10.1016/j.tetlet.2008.06.095)
a protocol for scalable reductive amination using an easily
metered liquid pyridine-borane complex. Mohammad Movassaghi of MIT devised
(Angew. Chem. Int. Ed. 2008, 47, 8909.
DOI: 10.1002/anie.200802921)
a strategy for conversion of an allylic carbonate 8 by way of the
allylic diazene to the terminal alkene 9.
Philippe Compain of the Université d’Orleans uncovered
(J. Org. 224295-73-2 Purity Chem. 2008, 73, 8647.
DOI: 10.1021/jo801626v)
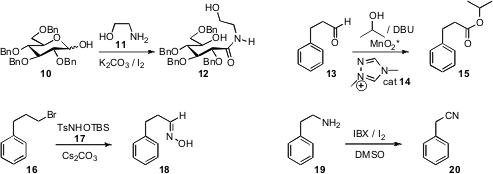
a practical procedure for oxidizing an inexpensive
aldose such as 10 to the amide 12, a valuable chiral pool starting
material. Karl A. Scheidt of Northwestern University extended
(Org. Lett. 2008, 10, 4331.
DOI: 10.1021/ol8018488)
activated MnO2 oxidation to saturated aldehydes such as
13, leading to the ester 15. Tohru Fukuyama of the University of Tokyo showed
(Org. Lett. 2008, 10, 2259.
DOI: 10.1021/ol800677p)
that halides such as 16 could be oxidized to the oxime 18 with the
reagent 17. The product oximes are readily dehydrated to the corresponding nitriles. Chutima Kuhakarn of Mahidol University devised
(Synthesis 2008, 2045.
DOI: 10.1055/s-2008-1067123)
a simple protocol for the oxidation of a primary amine such as 19 to the
nitrile 20.
Nasser Iranpoor and Habib Firouzabadi of Shiraz University developed
(J. Org. Chem. 2008, 73, 4882.
DOI: 10.1021/jo8000782)
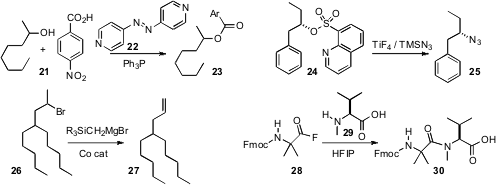
the reagent 22 for Mitsunobu coupling. The stereochemical course of this
reaction with simple acyclic secondary alcohols such as 21 was not reported.
Salvatore D. Lepore of Florida Atlantic University optimized
(Angew. Chem. Int. Ed. 2008, 47, 7511.
DOI: 10.1002/anie.200802472)
the quisylate 24 for the displacement with retention to
give the azide 25. Hideki Yorimitsu and Koichiro Oshima of Kyoto
University optimized
(J. Am. Chem. Soc. 2008, 130, 11276.
DOI: 10.1021/ja804277x)
a Co catalyst for the conversion of a secondary halide such as 26 to the
terminal alkene 27. Base-mediated elimination gave primarily the internal
alkene. Christian E. Schafmeister of the University of Pennsylvania established
(J. Am. Chem. Soc. 2008, 130, 14382.
DOI: 10.1021/ja806063k)
that acyl fluorides such as 28 couple efficiently even with unreactive
amino acids such as 29.
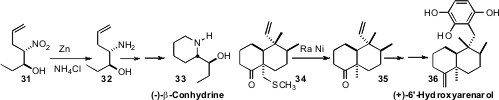
In the course of a synthesis of (-)-β-conhydrine (33)
(Tetrahedron Lett. 2008, 49, 6508.
DOI: 10.1016/j.tetlet.2008.08.113),
Nabin C. Barua of North East Institute of Science and Technology needed to
reduce the nitro group of 31 to the amine without reducing the very reactive
monosubstituted alkene. Zn/NH4Cl served well. James C. Anderson of
the University of Nottingham solved
(J. Org. Chem. 2008, 73, 8033.
DOI: 10.1021/jo801404u)
a similar problem in a synthesis of (+)-6′-hydroxyarenarol (36). In that case,
Raney Ni reduced the carbon-sulfur bond without affecting the monosubstituted alkene.