Yujiro Hayashi of Tokyo University of Science and Teruaki Mukaiyama of the
Kitasato Institute developed
(Chem. Lett. 1220039-63-3 Chemical name 2008, 37, 592
DOI: 10.1246/cl.2008.592)
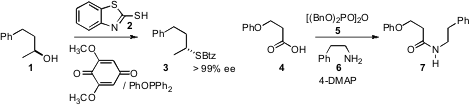
a reduction-oxidation method for converting primary, secondary (such as 1,
with clean inversion) and tertiary alcohols to sulfides. Peter A. Crooks of the
University of Kentucky found
(Chem. Lett. 2008, 37, 528
DOI: 10.1246/cl.2008.528)
that tetrabenzylpyrophosphate 5 was an effective agent for condensing an
acid 4 with an amine 6 to give the amide 7. This protocol,
that runs in near quantitative yield in an hour at room temperature, with all
impurities readily removable by washing with aqueous base and aqueous acid,
appears to be well-suited both for scale-up, and for solid-phase synthesis.
Balchandra M. PMID:23329319 Bhanage of the University of Mumbai reported
(Tetrahedron Lett. 2008, 49, 965
DOI: 10.1016/j.tetlet.2007.12.023)
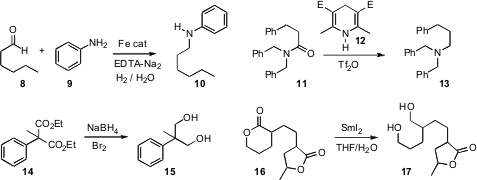
the reductive amination of aldehydes,
including 8, and ketones to the corresponding amines, using
H2
and an inexpensive Fe catalyst. André Charette of the Université de Montréal
showed (J. Am. Chem. Price of 173841-05-9 Soc. 2008, 130, 18
DOI: 10.1021/ja077463q)
that the Hantzsch ester 12, in the presence of Tf2O, reduced amides selectively
to amines. Esters, epoxides, ketones, nitriles and alkynes were stable to these
conditions.
Matthew Tudge of Merck Rahway demonstrated
(Tetrahedron Lett. 2008, 49, 1041
DOI: 10.1016/j.tetlet.2007.12.001)
that Br2 in DME activated
NaBH4, allowing
facile reduction of esters, including the congested diester 14, at
ambient temperature. David J. Procter of the University of Manchester made
(J. Am. Chem. Soc. 2008, 130, 1136
DOI: 10.1021/ja078137d)
the remarkable observation that six-membered ring
lactones such as 16
were reduced to the corresponding diol with
SmI2. Five-membered ring
and seven-membered ring lactones were not reduced under these conditions.
Bruce H. Lipshutz of the University of California, Santa Barbara devised
(Org. Lett. 2008, 10, 289
DOI: 10.1021/ol702689v)
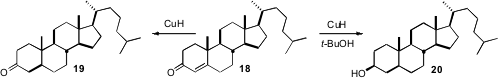
a convenient and economical procedure for
CuH, using Cu and an inexpensive
ligand in catalytic amounts, with PMHS as the bulk reductant. The reduction
of 18 presumably proceeds by electron transfer, as with dissolving metal
reduction, delivering 19 with the more
stable trans ring fusion. In the presence of t-BuOH as a proton source,
the reduction goes on to the alcohol 20. This may be the current method
of choice for selectively reducing a cyclohexanone to the equatorial alcohol.
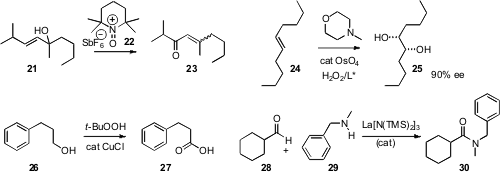
It is well known that tertiary allylic alcohols such as 21 can be
oxidized to the corresponding enone 23 with chromium reagents. Yoshiharu
Iwabuchi of Tohoku University observed
(J. Org. Chem. 2008, 73, 4750
DOI: 10.1021/jo800634r)
that the oxammonium salt 22 derived from
TEMPO effected the same
transformation. David E. Richardson of the University of Florida found
(Tetrahedron Lett. 2008, 49, 1071
DOI: 10.1016/j.tetlet.2007.11.186)
that H2O2 could be used to oxidize N-methylmorpholine
in situ to the N-oxide, that in turn reoxidized catalytic
OsO4.
In the presence of the Sharpless ligand, the
dihydroxylation proceeded with high
ee. This approach could offer cost and waste stream advantages over currently
used oxidants.
G. Sekar of the Indian Institute of Technology Madras, in Chennai,
established (Tetrahedron Lett. 2008, 49, 2457
DOI: 10.1016/j.tetlet.2008.02.031)
a convenient procedure for oxidizing primary alcohols such as 26 to the acid 27.
Secondary alcohols were oxidized to ketones. Allylic and benzylic alcohols could
be oxidized in preference to saturated alcohols. Tobin J. Marks of Northwestern
University devised
(Org. Lett. 2008, 10, 317
DOI: 10.1021/ol702788j)
a La catalyst for the oxidative amination of aldehydes. In its present incarnation, excess
aldehyde served as the reductant. If a less expensive reductant could be found,
this would be a very useful procedure, avoiding the carboxylic acid activation
usually required for amide formation.