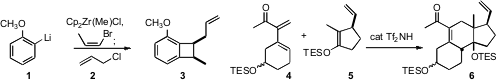
José Barluenga of the Universidad de Oviedo described(Org. Lett. 2008, 10, 4469.DOI: 10.1021/ol801652h)a powerful route from lithiated arenes such as 1 to the benzocyclobutane 3, the immediate precursor to the powerfulo-quinone methide Diels-Alder diene. Michael E. Jung of UCLA developed(Org. Lett. 2008, 10, 3647.DOI: 10.1021/ol801426z)a triflimide catalyst for the inverse electron demand coupling of thehighly substituted diene 4 with the enol ether 5 to give 6 with high diastereocontrol.
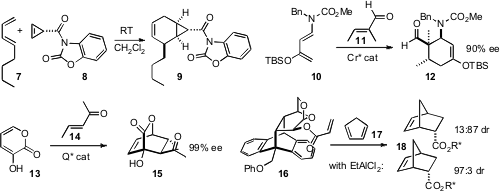
Joseph M. Fox of the University of Delaware showed(J. Org. Chem. 2008, 73, 4283.DOI: 10.1021/jo800042w)that the cyclopropene carboxylate 8 was a powerful and selective dienophile.Richard P. PMID:24275718 Mal-PEG3-NHS ester site Hsung and Kevin P. Cole of the University of Wisconsin finally(Adv. Synth. Catal. 76947-02-9 Price 2008, 350, 2885.DOI: 10.1002/adsc.200800552)reduced to practice the long-sought enantioselectiveDiels-Alder cycloaddition of a trisubstituted aldehyde, 11.Li Deng of Brandeis University devised(J. Am. Chem. Soc. 2008, 130, 2422.DOI: 10.1021/ja078251w)a Cinchona-derived catalyst for Diels-Alder cycloaddition to the diene 13 with high ee. Miguel Á. Sierra of the Universidad Complutense, Madrid,and Alejandra G. Suárez of the Universidad Nacional de Rosario designed(Org. Lett. 2008, 10, 3389.DOI: 10.1021/ol801140g)a clever switchable chiral auxiliary 16 that favored diastereomer S–18 on thermal addition, but R–18 with EtAlCl2.
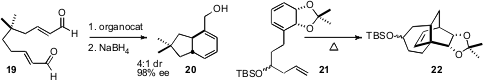
New approaches to the intramolecular Diels-Alder reaction continue tobe introduced. Mathias Christmann of TU Dortmund showed(Angew. Chem. Int. Ed. 2008, 47, 1450.DOI: 10.1002/anie.200704688)that a secondary amine organocatalyst converted the prochiral dialdehyde 19 into the bicyclic diene 20 with high de and ee. Martin G. Banwell of the Australian National University prepared(Org. Lett. 2008, 10, 4465.DOI: 10.1021/ol801647h)the triene 21 in high ee by microbiological oxidation of iodobenzene. On warming, 21 was converted smoothly into 22, which was carried on in a formal synthesis of platencin.
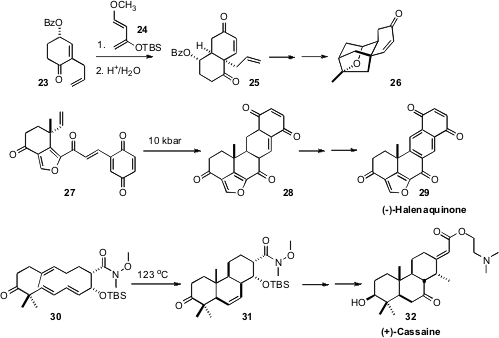
Jun-ichi Matsuo of Kanazawa University was able(Org. Lett. 2008, 10, 4049.DOI: 10.1021/ol801584r)to induce (neat, 180°C) the intermolecular Diels-Alder cycloaddition of 23 with 24, delivering the cycloadduct 25 with 11:1 diastereocontrol. This set the two rings and angular substitution of the key platensimycin intermediate 26. Dirk Trauner, now at the University of Munich, found(J. Am. Chem. Soc. 2008, 130, 8604.DOI: 10.1021/ja8035042)that the intramolecular Diels-Alder cyclization of 27 was most efficient at high pressure. The product 28 was readily tautomerized and then oxidized to give (-)-Halenaquinone 29. Pierre Deslongchamps of the Université de Sherbrooke extensively investigated(J. Am. Chem. Soc. 2008, 130, 13989.DOI: 10.1021/ja805097s)the transannular Diels-Alder cyclization of 30 and derivatives. The adduct 31 was carried on to (+)-cassaine 32.