As new drug entities must be usually be prepared as single enantiomers, and as many contain one or more heterocyclic or carbocyclic rings, there is an increased emphasis on the development of practical methods for the construction of enantiomerically pure cyclic systems. In this three-part series, we will cover the most important recent advances. (Part One)
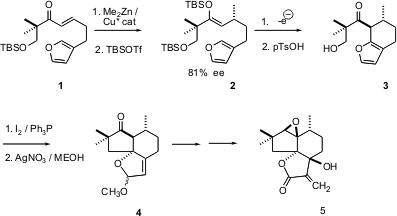
Enantiomerically-pure ternary stereogenic centers, often readily available, can be used to set the helicity of ring-forming reactions, leading to diastereomerically- and so enantiomerically-pure products. 4-Bromo-5-fluoro-2-methylpyridine In stock In the course of a synthesis of the antifungal sesquiterpene (-)-alliacol A5, Kevin Moeller of Washington University (J. Am. PMID:26895888 Chem. 91115-01-4 manufacturer Soc. 2004,126, 9106.DOI: 10.1021/ja048085h)set the absolute configuration of 2 by chiral catalytic conjugate addition to the prochiral1. Electrochemical oxidation of 2 gave 3, which was cyclized to4. Further manipulation of 4 gave 5.
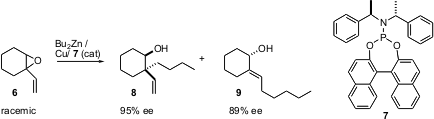
Another approach to the preparation of enantiomerically-pure ring systems is to start with the prochiral preformed ring, and perform an asymmetric transformation. Mauro Pineschi of the Università di Pisa has reported (J. Org. Chem. 2004, 69, 2099.DOI: 10.1021/jo035674h)a study of a series of racemic cyclic epoxides, represented here by6. Exposure of 6 to Bu2Zn in the presence of an enantiomerically-pure Cu complex derived from 7 converts one enantiomer of 6 to 8, and the other enantiomer to9, each in high enantiomeric excess. Several other classes of cyclic epoxides showed similar selectivity.
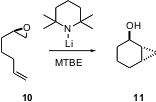
Epoxides such as 10 can be prepared in high enantiomeric purity, by, inter alia, kinetic resolution. David Hodgson of Oxford University has demonstrated (J. Am. Chem. Soc. 2004, 126, 8664.DOI: 10.1021/ja047346k)that on exposure to LTMP, monosubstituted epoxides are smoothly converted into the corresponding alkoxy carbene or alkoxy carbenoid. When an alkene is available for insertion, the cyclopropane, in this case11, is formed with high diastereocontrol. This represents a powerful new approach to enantioselective ring construction. It is possible that in the absence of a target alkene, the intermediate alkoxy carbene could divert to intramolecular C-H insertion, which might also proceed with substantial diastereocontrol.