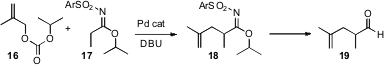Several remarkable one-carbon homologations have recently appeared. André B. 1228281-54-6 Data Sheet
Charette of the Université de Montréal reported
(J. 2869955-58-6 Formula Org. Chem. 2008, 73, 8097.
DOI: 10.1021/jo8014616)
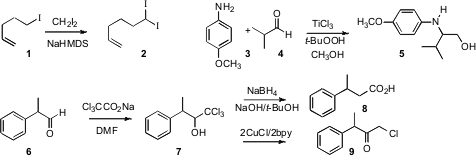
the alkylation of diiodomethane with alkyl iodides such as 1,
to give the diiodoalkane 2. Carlo Punta and the late Ombretta Porta of
the Politecnico di Milano effected
(Org. Lett. 2008, 10, 5063.
DOI: 10.1021/ol802244n)
reductive condensation of an amine 3 with an aldehyde 4 in the
presence of methanol, to give the amino alcohol 5. Timothy S. Snowden of
the University of Alabama showed
(Org. Lett. 2008, 10, 3853.
DOI: 10.1021/ol8016484)
that
NaBH4 reduced the carbinol 7, easily prepared from
the aldehyde 6, to the acid 8. Ram N. Ram of the Indian Institute
of Technology, Delhi found
(J. Org. Chem. 2008, 73, 5633.
DOI: 10.1021/jo8007644)
that CuCl reduced 7 to the chloro ketone 9.
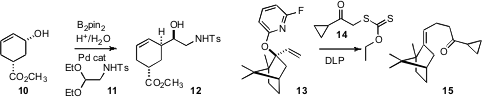
Kálmán J. Szabó of Stockholm University extended
(Chem. Commun. PMID:35901518 2008, 3420.
DOI: 10.1039/b804920c)
his elegant work on in situ borinate formation, coupling, in one
pot, the allylic alcohol 10 with the acetal 11 (hydrolysed in situ)
to deliver the alcohol 12 as a single diastereomer. Samir Z. Zard of the
Ecole Polytechnique developed
(J. Am. Chem. Soc. 2008, 130, 8898.
DOI: 10.1021/ja802899m)
the 6-fluoropyridyloxy ether of 13 as an effective radical leaving
group, enabling efficient coupling with 14, activated by dilauroyl
peroxide, to give 15.
Shu Kobayashi of the University of Tokyo established
(Chem. Commun. 2008, 6354.
DOI: 10.1039/b815845b)
that the anion of the sulfonyl imidate 17 participated in
direct Pd-mediated allylic coupling with the carbonate 16. The product
sulfonyl imidate 18 is itself of medicinal interest. It is also easily
converted to other functional groups, including the aldehyde 19.
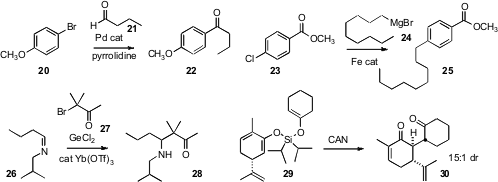
Jianliang Xiao of the University of Liverpool found
(J. Am. Chem. Soc. 2008, 130, 10510.
DOI: 10.1021/ja804351z)
that Pd-mediated coupling of an aldehyde 21
in the presence of pyrrolidine led to the ketone 22. The reaction is
probably proceeding via Heck coupling of the aryl halide with the in situ
generated enamine. Alois Fürstner of the Max Planck Institut, Mülheim observed
(J. Am. Chem. Soc. 2008, 130, 8773.
DOI: 10.1021/ja801466t)
that in the presence of the
simple catalyst Fe(acac)3 a
Grignard reagent 24 coupled
smoothly with an aryl halide 23 to give 25. Akio Baba of Osaka
University established
(Angew. Chem. Int. Ed. 2008, 47, 6620.
DOI: 10.1002/anie.200800194)
that the GeCl2-driven
Reformatsky-like addition of a halo
ketone 27 to an imine 26 worked best in the presence of a
catalytic Lewis acid.
The oxidative cross-coupling of ketone enolates, leading to the 1,4-diketone,
has great potential as a method for rapidly assembling molecular complexity.
Attempted hetero cross-coupling, however, can also lead to the homo coupled
products as contaminants. Regan J. Thomson of Northwestern University has now shown
(Org. Lett. 2008, 10, 5621.
DOI: 10.1021/ol802516z)
that it is possible to first prepare the hetero-coupled mixed silyl enol ethers, such
as 29. Oxidation of 29 delivered 30 as predominantly a single diastereomer.