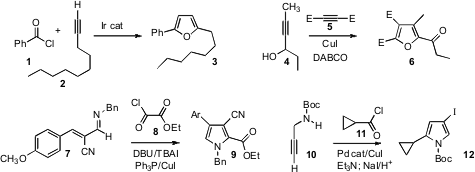
It has been known for some time that an acid chloride 1 can be added to an
alkyne 2 to give the β-chloro enone. Yasushi Tsuji of Kyoto University found
(J. Am. Chem. Soc. PMID:23310954 2009, 131, 6668.
DOI: 10.1021/ja901778y)
that with an Ir catalyst, the condensation of 1 with 2 could be directed to
the furan 3. Huanfeng Jiang of the South China University of Technology described
(Org. Lett. 2009, 11, 1931.
DOI: 10.1021/ol900364y)
a complementary route to furans, Cu-mediated condensation of a propargyl alcohol
4 with the diester 5 to give 6.
Bruce A. Arndtsen of McGill University developed
(Org. Lett. 2009, 11, 1369.
DOI: 10.1021/ol900185n) an approach to
pyrroles such as 9, by condensation of an α,β-unsaturated α-cyano
imine 7 with the acid chloride 8.
Thomas J. 1196145-01-3 web J. BuyMethyltrioxorhenium(VII) Müller of Heinrich-Heine-Universität Düsseldorf observed
(Org. Lett. 2009, 11, 2269.
DOI: 10.1021/ol900581a)
the condensation of an acid chloride 11 with a propargyl amine 10,
leading to the iodo pyrrole 12.
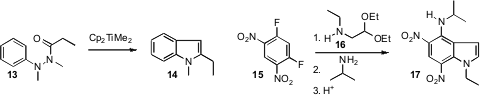
John A. Murphy of the University of Strathclyde uncovered
(Tetrahedron Lett. 2009, 50, 3290.
DOI: 10.1016/j.tetlet.2009.02.060)
a new entry to the Fischer indole synthesis, by Petasis homologation of a
hydrazide 13. Dali Yin of Peking Union Medical College took advantage
(Org. Lett. 2009, 11, 637.
DOI: 10.1021/ol8027187)
of the easy sequential displacement of the fluorides of 15,
leading, after acid-catalyzed cyclization, to the
indole
17. Kang Zhao of Tianjin University extended
(Org. Lett. 2009, 11, 2417,
DOI: 10.1021/ol900576a
; Org. Lett. 2009, 11, 2643,
DOI: 10.1021/ol9006663)
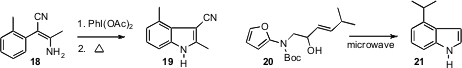
his studies of oxidation of an enamine
18 to the
2H-azirine, that on heating cyclized to the indole 19. Peter Wipf of the
University of Pittsburgh established
(Chem. Commun. 2009, 104.
DOI: 10.1039/b816989f)
a microwave-promoted indole synthesis, illustrated by the intramolecular
Diels-Alder cyclization of 20 to 21. A review delineating all nine types of
indole syntheses will appear shortly in Angewandte Chemie.
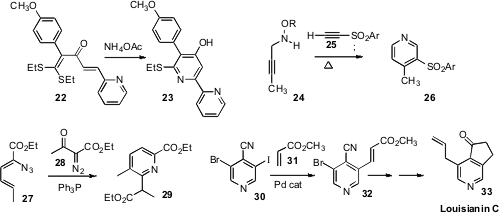
Fushun Liang and Qun Liu of Northeast Normal University demonstrated
(J. Org. Chem. 2009, 74, 899.
DOI: 10.1021/jo802318p)
that the readily-prepared ketene thioacetal 22 condensed
with NH3 to give the
pyridine 23. Sundaresan Prabhakar and Ana M. Lobo of the
New University of Lisbon observed
(Tetrahedron Lett. 2009, 50, 3446.
DOI: 10.1016/j.tetlet.2009.02.228)
that the addition of the alkoxy propargyl amine to the alkyne 25 gave a Z alkene, that on
warming rearranged to the pyridine 26. Yan-Guang Wang of Zhejiang University, Hangzhou developed
(J. Org. Chem. 2009, 74, 903.
DOI: 10.1021/jo802159g)
a pyridine synthesis based on the
Wolff rearrangement of a diazo ketone such as 28.
The iminophosphorane derived from 27 added to the resulting ketene, leading, after
by electrocyclic rearrangement, to the pyridine 29.
A great deal of work has been done on the selective metalation of pyridines.
Ching-Yao Chang of Asia University, Taichung used this
(Tetrahedron 2009, 65, 748,
DOI: 10.1016/j.tet.2008.11.075); for another report on pyridine metalation, see
Tetrahedron Lett. 2009, 50, 1768,
DOI: 10.1016/j.tetlet.2008.12.068)
to advantage in developing a synthetic route to the Streptomyces-derived
Louisianin alkaloids. Lithiation of 4-cyanopyridine was followed by bromination.
The product was again lithiated, then iodinated to give 30. A selective
Heck reaction on 30 gave 32, that was carried on to Louisianin C (33).