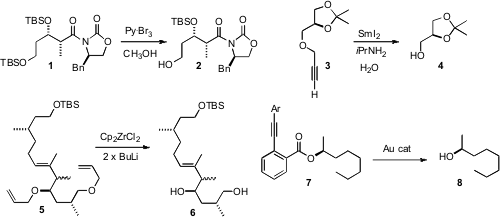
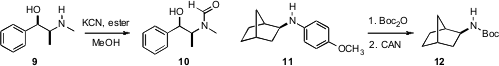Alcohols are usually protected as alkyl or silyl ethers. PMID:23626759 Michael P. Jennings
of the University of Alabama found
(Tetrahedron Lett. 2008, 49, 5175
DOI: 10.1016/j.tetlet.2008.06.072)
that pyridinium tribromide can selectively
remove the TBS (or TES)
protection from the primary alcohol of a protected primary-secondary alcohol
such as 1. Propargyl ethers are useful because they are stable, but can
be selectively removed in the presence of other protecting groups. Shino Manabe
and Yukishige Ito at RIKEN showed
(Tetrahedron Lett. 2008, 49, 5159
DOI: 10.1016/j.tetlet.2008.06.081)
that SmI2 could reductively remove a propargyl group in the
presence of acetonides (illustrated, 3), MOM, benzyl and TBS ethers.
Hisanaka Ito of the Tokyo University of Pharmacy and Life Sciences took advantage
(Org. Price of Apixaban Lett. 4-Amino-2-fluoro-5-methoxybenzoic acid Formula 2008, 10, 3873
DOI: 10.1021/ol801395q)
of the reducing power of Cp2Zr to selectively remove the allyl ethers
from 5, to give 6. These conditions might also remove propargyl ethers.
Esters can also be useful protecting groups. Naoki Asao of Tohoku University developed
(Tetrahedron Lett. 2008, 49, 7046
DOI: 10.1016/j.tetlet.2008.09.146)
the o-alkynyl ester 7. Au catalyst in EtOH removed the ester,
leaving benzoates, acetates, OTBS and OTHP intact. Alternatively, an o-iodobenzoate
can be removed by Sonogashira coupling followed by the Au hydrolysis.
N-Formylation is usually accomplished using mixed anhydrides. Weige Zhang and
Maosheng Chang of Shenyang Pharmaceutical University put forward
(Chem. Commun. 2008, 5429)
DOI: 10.1039/b810086a)
an intriguing alternative, heating a secondary
amine 9 with KCN in the presence of dimethyl malonate to give 10.
Many of the current methods for amination that have been developed deliver
the aryl amine. John F. Hartwig of the University of Illinois established
(J. Am. Chem. Soc. 2008, 130, 12220
DOI: 10.1021/ja803523z)
that exposure of the amine
11 to Boc2O followed by CAN led to the protected, dearylated
amine 12.
Adam McCluskey of The University of Newcastle observed
(Tetrahedron Lett. 2008, 49, 6962
DOI: 10.1016/j.tetlet.2008.09.027)
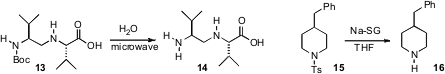
that microwave heating removed Boc protecting
groups when there was a free carboxylic acid elsewhere in the molecule. Michael
Lefenfeld of SiGNa Chemistry and James E. Jackson of Michigan State University used
(Org. Lett. 2008, 10, 5441
DOI: 10.1021/ol8021337)
easily-handled Na/silica gel to remove primary and secondary sulfonamides
(e.g. 15 → 16). Methanesulfonamides were also removed under these conditions.
Carboxylates are good SN2 nucleophiles. Santos Fustero of the
Universidad de Valencia took advantage of this
(J. Org. Chem. 2008, 73, 5617
DOI: 10.1021/jo800567a)
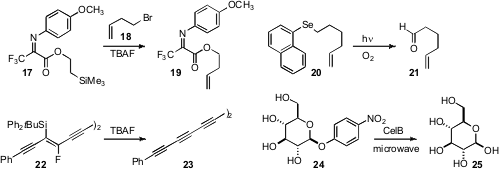
in developing a transesterification of TMSE esters. Exposure of
17 to TBAF in the presence of 18 gave 19. Akihiko Ouchi of
the University of Tsukuba showed
(J. Org. Chem. 2008, 73, 8861
DOI: 10.1021/jo801730j)
that an aryl selenide such as 20 could be unmasked by
photo-oxygenation to give the corresponding aldehyde 21. Secondary
selenides gave ketones. Liam R. Cox of the University of Birmingham converted
(Tetrahedron Lett. 2008, 49, 4596
DOI: 10.1016/j.tetlet.2008.05.081)
halosilanes such as 22 to the corresponding alkyne 23 by exposure to TBAF.
There has been much discussion about the exact role microwaves play in
promoting organic reactions. It is clear that microwaves can activate peptide
bond rotation. This may be a factor in the observation
(J. Am. Chem. Soc. 2008, 130, 10048
DOI: 10.1021/ja802404g)
by Alexander Deiters of North Carolina State
University that the rate of the hydrolysis of 24 to 25 by the β-glucosidase
CelB from the hyperthermophilic archaeon Pyrococcus furiosus increased by at
least four orders of magnitude under microwave irradiation.