(+)-Bruguierol A (Fañanás/Rodríguez), (-)-Berkelic Acid (Snider), and (-)-Aigialomycin
D (Harvey)
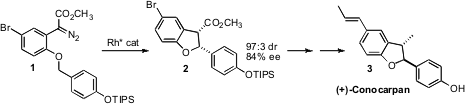
(+)-Conocarpan (3), isolated from the wood of Conocarpus erectus,
exhibits insecticidal, antifungal and antitrypanosomal activity. Shunichi
Hashimoto of Hokkaido University developed
(J. Org. Chem. 2009, 74, 4418.
DOI: 10.1021/jo900502d)
a chiral Rh (II) carboxylate that effected the cyclization of 1
to 2, setting the absolute configuration of 3.
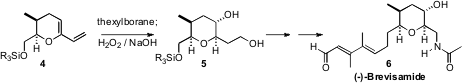
The dinoflagellate Karenia brevis produces the brevetoxins, a family
of complex polyethers. Recently, the first N-containing
cyclic ether, (-)-Brevisamide
(6), was isolated from K. brevis. Masayuki Satake and Kazuo
Tachibana of the University of Tokyo, in their synthesis of 6
(Org. 935845-20-8 Price Ruphos pd(crotyl)cl Order Lett. PMID:24883330 2009, 11, 217.
DOI: 10.1021/ol802426v)
found it convenient to set the relative configuration around the six-membered
ring by double hydroboration/oxidation of the diene 4.
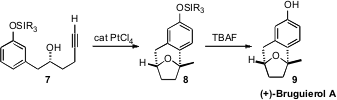
(+)-Bruguierol A (9), isolated from the mangrove Bruguiera
gymmorrhiza, has an unusual bridged structure. Francisco J. Fañanás and
Félix Rodríguez of the Universidad de Oviedo conceived
(J. Org. Chem. 2009, 74, 932.
DOI: 10.1021/jo8021204)
an elegant approach to the construction of 9,
based on the Pt-mediated addition of the alcohol of 7 to the alkyne to
give a transient enol ether. It is not clear whether the subsequent
intramolecular electrophilic addition to the aromatic ring is mediated by the
Pt, or by a trace of adventitious acid. The overall transformation was
remarkably efficient.
The Berkeley Pit in Butte, Montana, is an abandoned open-pit copper mine
filled with 30 billion gallons of pH = 2.5 water heavily contaminated with,
inter alia, copper, cadmium, arsenic and zinc. Remarkably, microorganisms can be
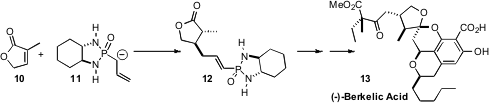
cultured from that water. (-)-Berkelic Acid (13), isolated from a
Penicillium fungus, showed selective activity against OVCAR-3 ovarian cancer.
Barry B. Snider of Brandeis University set
(Angew. Chem. Int. Ed. 2009, 48, 1283.
DOI: 10.1002/anie.200805488)
the absolute configuration of the central five-membered ring
ether of 13 by conjugate addition of the enantiomerically-pure reagent
11 to the prochiral lactone 10.
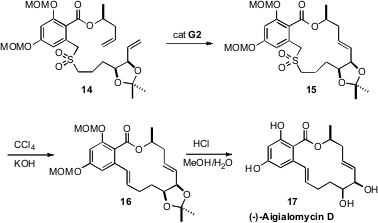
(-)-Aigialomycin D (17), isolated from the mangrove fungus Aigialus
parvus, was found to be a selective inhibitor of the kinases CDK1, CDK5 and
GSK3. The synthesis of 17 reported
(J. Org. Chem. 2009, 74, 2271.
DOI: 10.1021/jo802561s)
by Joanne Harvey of Victoria University of Wellington illustrated
the power of the Ramberg-Bäcklund reaction for the closure of medium rings.
Three-component coupling allowed the facile assembly of the sulfone 14.
Ring-closing metathesis gave 15, that on oxidative SO2
extrusion followed by deprotection gave (-)-Aigialomycin D (17).