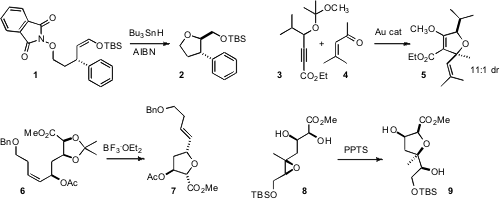
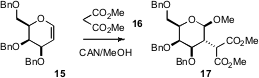O-Centered radicals have been little used for C-O ring formation. PMID:24633055 Glenn M.
Sammis of the University of British Columbia showed
(Org. Lett. 2008, 10, 5083
DOI: 10.1021/ol802142k)
that O-centered radicals could be generated efficiently, and
that they cyclized with high diasterecontrol. Liming Zhang of the University of
Nevada, Reno, continuing his studies of Au-activation of alkynes, uncovered
(J. 1-Methylcyclopropaneacetic acid Formula Am. Chem. Soc. 2008, 130, 12598
DOI: 10.1021/ja804690u)
the bimolecular condensation of polarized alkynes such as 3 with
aldehydes and ketones, including 4, to give the
dihydrofuran with
high diastereocontrol.
Margarita Brovetto of the Universidad de la República, Montevideo, Uruguay, prepared
(J. Org. Chem. 2008, 73, 5776
DOI: 10.1021/jo800514k)
the precursor to the enantiomercially triol 6 by fermentation of bromobenzene with
Pseudomonas putida 39/D. Cyclization of 6 gave 7 with high
diastereocontrol. Petri M. Pihko of the University of Jyväskylä, Finland, found
(Org. Lett. 31420-52-7 web 2008, 10, 4179
DOI: 10.1021/ol8015868)
that cyclization of 8,
prepared by Sharpless asymmetric epoxidation followed by
Sharpless asymmetric
dihydroxylation, also proceeded with high diastereocontrol.
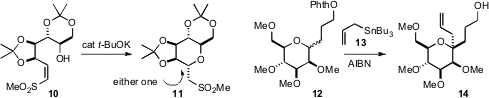
Vincent Aucagne of the Université d’Orléans observed
(Tetrahedron Lett. 2008, 49, 4750
DOI: 10.1016/j.tetlet.2008.05.117)
that brief exposure of the sulfone 10 to
t-BuOK at low temperature gave clean conversion to the kinetic diastereomer
11. At room temperature, similar conditions delivered the other, more
stable diastereomer. Angeles Martín and Ernesto Suárez of the C. S. I. C., La
Laguna, took advantage
(Tetrahedron Lett. 2008, 49, 5179
DOI: 10.1016/j.tetlet.2008.06.070)
of the facile generation of O-centered radicals in converting 12 to 14,
having a stereocontrolled quaternary center. The transformation is thought to be
proceeding by H-atom abstraction, then diastereocontrolled trapping of the
C-radical so formed with the allyl stannane 13.
Much of the effort toward alkylated
cyclic ether construction has been
focused on alkyl group attachment adjacent to the ring oxygen. Torsten Linker of
the University of Potsdam developed
(J. Am. Chem. Soc. 2008, 130, 16003
DOI: 10.1021/ja8052706)
a complementary approach, stereocontrolled oxidative radical
addition of malonate 16 to glycals such as 15 to give the 3-alkyl
substituted 17.
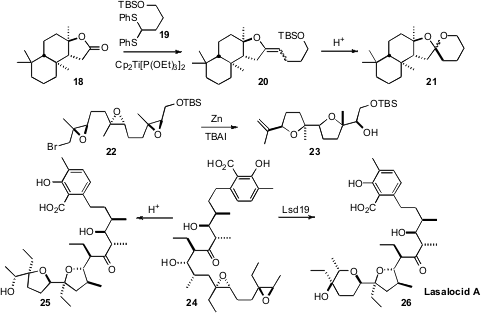
Richard C. Hartley of the University of Glasgow established
(Tetrahedron Lett. 2008, 49, 4771
DOI: 10.1016/j.tetlet.2008.05.094)
what promises to be a powerful strategy
for the convergent assembly of spiro ketals, based on the condensation of the Ti
alkylidene derived from a thioacetal such as 19 with a lactone such as
18. James A. Marshall of the University of Virgina nicely reduced to practice
(J. Org. Chem. 2008, 73, 6753
DOI: 10.1021/jo801188w)
the preparation and reductive cyclization of polyepoxides such as 22. Hiroki
Oguri and Hideaki Oikawa of Hokkaido University demonstrated
(J. Am. Chem. Soc. 2008, 130, 12230
DOI: 10.1021/ja8040543)
that overexpressed enzyme Lsd19 converted 24
to lasolacid A (26). With acid, 24 cyclized to 25.