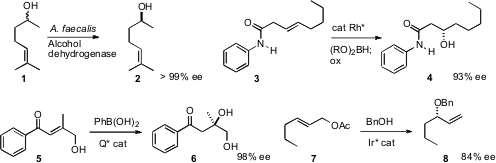
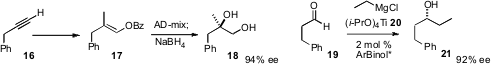Enzymatic reduction of a ketone can proceed in high enantiomeric excess, but
this would require a stoichiometric amount of a reducing agent. Wolfgang Kroutil
of the Karl-Franzens-Universität Graz devised
(Angew. Chem. Int. Ed. 2008, 47, 741
DOI: 10.1002/anie.200703296)
a protocol for preparing the alcohol 2 in high ee starting from the racemic
alcohol. The alcohol dehydrogenase chosen was selective for the R alcohol, and
the microorganism reduced the ketone so produced selectively to the S alcohol. 1212934-10-5 Formula
James M. Takacs of the University of Nebraska established
(J. 7-Bromochromane-3-carboxylic acid Price Am. Chem. Soc. 2008, 130, 3734
DOI: 10.1021/ja710492q)
that chiral Rh catalyzed addition of
pinacolborane to a β,γ-unsaturated
N-phenyl amide 3 proceeded with high enantiocontrol. The product organoborane
was oxidized to the alcohol 4. J. R. Falck of the UT Southwestern Medical Center used
(J. Am. Chem. PMID:24856309 Soc. 2008, 130, 46
DOI: 10.1021/ja076802c)
an organocatalyst to effect addition of
phenylboronic acid to the γ-hydroxy enone 5, to give, after hydrolysis, the diol
6. John F. Hartwig of the University of Illinois effectively telescoped
(Angew. Chem. Int. Ed. 2008, 47, 1928
DOI: 10.1002/anie.200705267)
alcohol formation and protection into a single
step, by developing a procedure for the direct conversion of a primary allylic
acetate 7 to the enantiomerically-enriched secondary
benzyl ether 8.
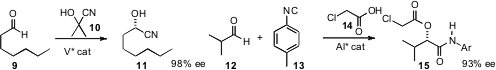
Tsutomu Katsuki of Kyushu University designed
(Chemistry Lett. 2008, 37, 502
DOI: 10.1246/cl.2008.502)
a catalyst for the enantioselective hydrocyanation of an aldehyde 9, by HCN
transfer from the inexpensive 10. Mei-Xiang Wang of the Chinese Academy of
Sciences, Beijing and Jieping Zhu of CNRS, Gif-sur-Yvette devised
(Angew. Chem. Int. Ed. 2008, 47, 388
DOI: 10.1002/anie.200704315)
a catalyst for a complementary one-carbon homologation,
the enantioselective Passerini three-component coupling of an aldehyde 12, an isonitrile
13, and an acid 14.
Joseph M. Ready, also of UT Southwestern, developed
(J. Am. Chem. Soc. 2008, 130, 7828
DOI: 10.1021/ja803480b)
the preparation of enol benzoates such as 17 from the corresponding
alkynes. Sharpless asymmetric dihydroxylation of 17 proceeded with high ee to
give, after reduction, the diol 18. Toshiro Harada of the Kyoto Institute of Technology described
(Angew. Chem. Int. Ed. 2008, 47, 1088
DOI: 10.1002/anie.200704963)
a potentially very practical enantioselective homologation, the catalyzed addition of an alkyl
titanium, prepared in situ from the corresponding Grignard reagent, to the
aldehyde 19, to give 21 in high ee.
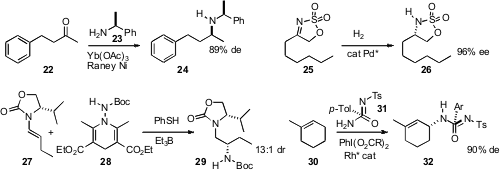
Thomas C. Nugent of Jacobs University Bremen reported
(J. Org. Chem. 2008, 73, 1297
DOI: 10.1021/jo7021235)
that added Yb(OAc)3 improved the de in the reductive amination of
ketones such as 22 with Raney Ni and 23. Yong-Gui Zhou of the
Dalian Institute of Chemical Physics found
(Org. Lett. 2008, 10, 2071
DOI: 10.1021/ol800591u)
that cyclic sulfamidates such as 25 were easily prepared from the corresponding
hydroxy ketone. Enantioselective hydrogenation of 25 gave 26 with high ee. Note
that the cyclic sulfamidates 26 so produced will be versatile intermediates for
further transformations, as the O is easily displaced by nucleophiles. Armido
Studer of Westfälische-Wilhelms-Universität has been investigating
(![]()
2008, March 24) the radical amination of alkenes. He has developed
(Angew. Chem. Int. Ed. 2008, 47, 779
DOI: 10.1002/anie.200703902) an
easily-prepared N donor, 28, and found that addition to the alkenyl
oxazolidinone 27 proceeded with high de.
The alkene 30 has ten chemically distinct C-H bonds. Paul Müller of the
University of Geneva and Robert H. Dodd and Philippe Dauban of CNRS,
Gif-sur-Yvette established
(J. Am. Chem. Soc. 2008, 130, 343
DOI: 10.1021/ja076519d)
that insertion by a Rh nitrene proceeded with high selectivity primarily into just one of those
C-H bonds, delivering 32 in high de.