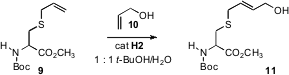Hervé Clavier and Steven P. Nolan, now at St. Andrew’s University, found (Adv. Synth. Catal. 2008, 350, 2959.DOI: 10.1002/adsc.200800495)that the indenylidene Ru complex 1 was an excellent pre-catalyst for alkene metathesis. A combination of 1 and the ligand 2 effectedcross metathesis of 3 and 4 in just 15 minutes undermicrowave heating. Robert H. Grubbs of Caltech designed(Org. 4-(Diphenylphosphino)phenol custom synthesis Lett. PMID:23865629 2008, 10, 2693.DOI: 10.1021/ol800824h)the Ru catalyst 6 for the preparation of tri- and tetrasubstituted alkenes, as illustrated by the conversion of 7 to 8. The catalyst6 also worked well for cross metathesis and ring opening metathesis polymerization (ROMP).
For some biological applications, it would be desirable to run alkene cross metathesisunder aqueous conditions. Benjamin G. Price of 2-chloro-4,6-dimethoxypyridine Davis of the University of Oxford observed(J. Am. Chem. Soc. 2008, 130, 9642.DOI: 10.1021/ja8026168)that allyl sulfides such as 9 were unusually reactive in cross metathesis. Indeed, aqeous cross methathesis with such an allyl sulfide incorporated in a protein worked well, although added MgCl2 was required. The protein, a serine protease, maintained its activity after cross metathesis.
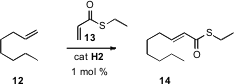
α,β-Unsaturated thioesters such as 14 are excellent substrates for, inter alia, enantioselective Cu-catalyzed conjugate addition of Grignard reagents. Adriaan J. Minnaard and Ben L. Feringa of the University of Groningen found(J. Org. Chem. 2008, 73, 5651.DOI: 10.1021/jo800879e)that the thioacrylate 13 was an excellent substrate for cross methathesis, allowing ready preparation of 14.
Although alkene metathesis is often run in CH2Cl2, benzene or toluene, these are not necessarily the optimal solvents. Siegfried Blechert of the TU Berlin established(Tetrahedron Lett. 2008, 49, 5968.DOI: 10.1016/j.tetlet.2008.07.161)that for the difficult cyclization of 16 to 17, hexafluorobenzene worked particularly well.
The extended conformation (illustrated for 18) of an ester is more stable than the lactone conformation by about 5 kcal/mol. It is therefore not surprising that SonBinh T. Nguyen of Northwestern University observed(Org. Lett. 2008, 10, 5613.DOI: 10.1021/ol8022227)that attempted ring-closing metathesis of 18 gave only the dimer20. On addition of the bulky Lewis acid 21, which can complex 18 in the lactone conformation, the reaction delivered the desired monomer19. This should be a generally useful strategy for the cyclization of difficult ester substrates.
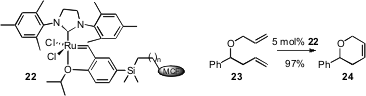
Su Seong Lee and Jackie Y. Ying of the Institute of Bioengineering and Nanotechnology, Singapore, constructed(Chem. Commun. 2008, 4312.DOI: 10.1039/b803663b)the metathesis catalyst 22, covalently bound to siliceous mesocellular foam. At 5% catalyst loading, the tenth cycle for the cyclization of 23 to 24 gave the same yield as the first cycle, 97%. Ru residues in solution were only 5-8 ppm.