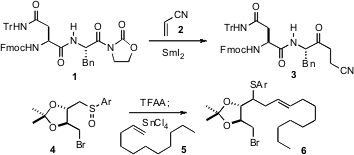
In the context of peptidyl ketone synthesis, Troels Skrydstrup of the
University of Aarhus developed
(J. Org. PMID:24377291 Chem. 2008, 73, 1088.
DOI: 10.1021/jo702286b)
the elegant SmI2-mediated conjugate addition of acyl
oxazolidinones such as 1 to acceptors such as 2. Sadagopan
Raghavan of the Indian Institute of Chemical Technology, Hyderabad reported
(Tetrahedron Lett. 2008, 49, 1601.
DOI: 10.1016/j.tetlet.2008.01.046)
that the addition of a Pummerer intermediate, generated by exposure of 4
to TFAA, to the terminal alkene 5 and SnCl4 led to efficient
C-C bond formation, to give the sulfide 6 as a single (unassigned) diastereomer.
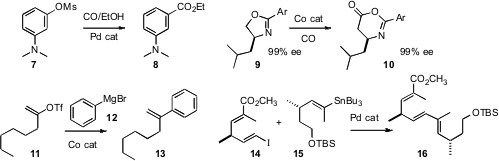
Pd-catalyzed carbonylation of aryl halides and triflates is a
well-established process. Stephen L. 5-Bromo-1,3-dihydroisobenzofuran Formula Buchwald of MIT has now
(J. Am. Chem. Soc. 2008, 130, 2754.
DOI: 10.1021/ja711449e)
extended this transformation to much less expensive tosylates and mesylates
such as 7. β-Amino acids have often been prepared from α-amino
acids by Arndt-Eistert homologation. Geoffrey W. Coates of Cornell University has devised
(Angew. Buy1-Bromobutan-2-one Chem. Int. Ed. 2008, 47, 3979.
DOI: 10.1002/anie.200705310)
a more practical alternative, the direct Co-catalyzed carbonylation of an
oxazoline 9 to the 2-oxazine-6-one 10.
Eiji Shirakawa and Tamio Hayashi of Kyoto University also used
(Chem. Lett. 2008, 37, 654.
DOI: 10.1246/cl.2008.654)
a Co catalyst to promote the coupling of aryl and alkenyl
Grignard reagents with enol
triflates such as 11. Alois Fürstner of the Max-Planck-Institut, Mülheim optimized
(Chem. Commun. 2008, 2873.
DOI: 10.1039/b805299a)
promoters for the Pd-catalyzed Stille-Migata coupling of iodo alkenes such as 14
with alkenyl stannanes such as 15 to give 16. It is particularly noteworthy
that their system is fluoride free.
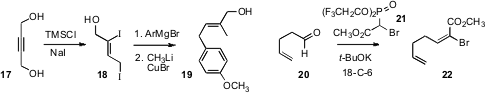
The stereocontrolled construction of trisubstituted alkenes continues to be
challenging. We described
(J. Org. Chem. 2008, 73, 1605.
DOI: 10.1021/jo7021197)
the facile preparation of the diioide 18 from the inexpensive 2-butyn-1,4-diol (17).
Sequential coupling of 18 with an aryl Grignard followed by CH3Li delivered 19.
Brian S. J. Blagg of the University of Kansas established
(Tetrahedron Lett. 2008, 49, 141.
DOI: 10.1016/j.tetlet.2007.10.150)
that Still-Genari homologation of 20 with 21 gave (E)-22 with high
geometric control. Biao Jiang of the Shangahi Institute of Organic Chemistry reported
(Org. Lett. 2008, 10, 593.
DOI: 10.1021/ol702859y)
a convenient alternative protocol to give (Z)-α-bromo unsaturated esters.
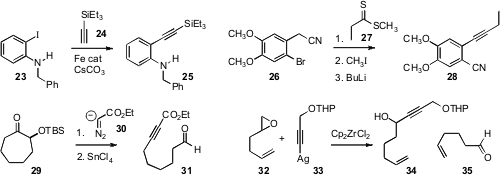
Carsten Bolm of RWTH Aachen University found
(Angew. Chem. Int. Ed. 2008, 47, 4862.
DOI: 10.1002/anie.200801539)
that an inexpensive Fe catalyst could replace Pd in the
Sonogashira coupling of 24 to 23.
Hiriyakkanavar Ila of the Indian Institute of Technology, Kanpur devised
(Org. Lett. 2008, 10, 965.
DOI: 10.1021/ol800029c)
a complementary route to aryl acetylenes, with concomitant ortho nitrile transfer.
Matthias Brewer of the University of Vermont (29 → 31,
J. Am. Chem. Soc. 2008, 130, 3766.
DOI: 10.1021/ja801004d)
and Gregory B. Dudley of Florida State University
(not illustrated, J. Am. Chem. Soc. 2008, 130, 5050.
DOI: 10.1021/ja801018r)
devised fragmentation schemes for alkyne construction. Kazunori Koide of the University of Pittsburgh found
(J. Org. Chem. 2008, 73, 1093.
DOI: 10.1021/jo702306k)
that exposure of an epoxide 32 to a silver acetylide 33 followed by stoichiometric
Cp2ZrCl2 and catalytic AgOTf led to alcohol 34. This is as though the epoxide
32 had rearranged to the aldehyde 35, a long-sought goal of organic synthesis.