G
Continuing efforts toward the direct functionalization of aromatic C-H bonds,
Nobutaka Fujii and Hiroaki Ohno of Kyoto University described
(Chem. Commun. 2009, 3413.
DOI: 10.1039/b905586j)
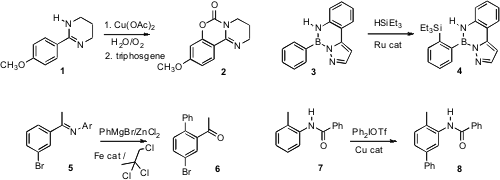
a Pd-mediated protocol for the ortho hydroxylation of
aryl tetrahydropyrimidines such as 1. To use a boronic acid as an
activating/directing group, Michinori Suginome, also of Kyoto University, devised
(J. Am. Chem. PMID:27108903 2460255-78-9 Data Sheet Soc. 2009, 131, 7502.
DOI: 10.1021/ja902314v)
the pyrazolylpyridyl derivative 3. The product 4 could be returned to
the boronic acid or carried on to the borate ester, in each case with recovery
of the directing group. Eiichi Nakamura of the University of Tokyo established
(Angew. Chem. Int. Ed. 2009, 49, 2925.
DOI: 10.1002/anie.200900454)
that ortho arylation of 5 could be accomplished even in the presence
of a reactive aryl halide. In a complementary approach, Matthew J. Gaunt of the
University of Cambridge developed
(Science 2009, 323, 1593.
DOI: 10.1126/science.1169975)
a procedure for C-H arylation of anilides such as 7 that showed good meta selectivity.
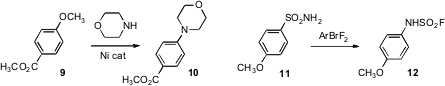
Mamoru Tobisu and Naoto Chatani of Osaka University have found
(Chem. (1S,2R)-2-Amino-1,2-diphenylethanol web Lett. 2009, 38, 710.
DOI: 10.1246/cl.2009.710)
for the conversion of aryl ethers such as 9
to the tertiary amine. Masahito Ochiai of the University of Tokushima observed
(J. Am. Chem. Soc. 2009, 131, 8392.
DOI: 10.1021/ja903544d)
the remarkable inversion of an arene sulfonamide such as 11 to the
protected aniline 12. Matthias Beller of the Universität Rostock established
(Angew. Chem. Int. Ed. 2009, 49, 918.
DOI: 10.1002/anie.200804898)
a Pd-mediated procedure for the conversion of even a
congested aryl halide 13 to the phenol 14.
The first C-C bond formations with phenols used very reactive, but expensive, leaving
groups such as triflates. With improving ligand design, conditions have been found
that work well even with inexpensive mesylates. Now, Zhang-Jie Shi of Peking University
(J. Am. Chem. Soc. 2008, 130, 14468.
DOI: 10.1021/ja8056503)
and Neil K. Garg of UCLA
(J. Am. Chem. Soc. 2008, 130, 14422.
DOI: 10.1021/ja806244b)
, working independently, developed similar procedures for the Ni-mediated
arylation of esters such as 15. Both groups found that pivalates worked
particularly well.
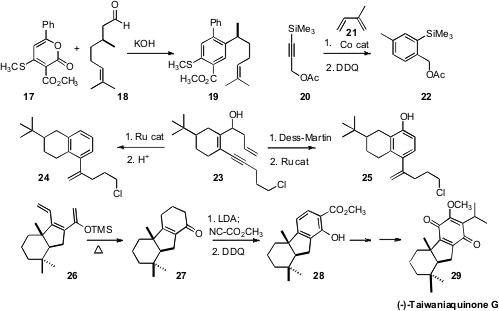
For some highly-substituted benzene derivatives, construction of the aromatic
ring can be an economical approach. Atul Goel of the Central Drug Research
Institute, Lucknow, found
(Tetrahedron Lett. 2009, 50, 2086.
DOI: 10.1016/j.tetlet.2009.02.117)
that the direct coupling of a pyrone such as 17 with an aldehyde 18
delivered the aryl ester 19. Gerhard Hilt of Philipps-Universität Marburg developed
(Org. Lett. 2009, 11, 773.
DOI: 10.1021/ol802837m)
Co catalysts for the Diels-Alder cyclization of 20 with 21, with
subsequent oxidation to the arene 22. With the proper choice of catalyst,
either regioisomer of the Diels-Alder adduct could be made to dominate. Kazuhiro
Yoshida and Akira Yanagisawa of Chiba University found
(J. Org. Chem. 2009, 74, 3632.
DOI: 10.1021/jo900456g)
a similar flexibility in a Ru-mediated arene construction. Cyclization of 23
followed by dehydration gave 24, while oxidation followed by cyclization delivered
the phenol 25. Enrique Alvarez-Manzaneda of the Universidad de Granada, in the course
(Chem. Commun. 2009, 592.
DOI: 10.1039/b816812a)
of a synthesis of (-)-Taiwaniaquinone G (29), built the
aromatic ring by cyclization of 26 to 27, followed by oxidation to
28.