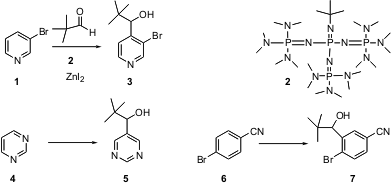
The efficient construction of substituted heterocycles is central to medicinal chemistry. Potassium tetrachloroplatinate(II) structure Yoshinori Kondo of Tohoku University reports (J. Am. PMID:23962101 Chem. Soc. 2003, 125, 8082.DOI: 10.1021/ja0342300)that the novel base 2 will, in the presence of ZnI2 and an aldehyde, deprotonate heterocycles, to give the hydroxyalkylated products 3 and 5. Benzene rings also participate – 6 is converted to7. Price of N-Methylsulfamoyl chloride
Free radical cyclizations have often been carried out with tin reagents, which are toxic, and an environmental hazard. As an alternative, phosphorous reagents have been developed, but these suffer from the shortcoming that they are aqueous, and so it is difficult to get them into contact with the organic substrate. John A. Murphy of the University of Strathclyde in Glasgow reports (Org. Lett. 2003, 5, 2971.DOI: 10.1021/ol035173i)that diethyl phosphine oxide (DEPO), which is soluble in organic solvents as well as in water, serves efficiently as a hydride source and radical mediator, smoothly cyclizing 8 to the indolone 9. The oxidized phosphonic acid byproduct is easily separated by aqueous extraction.
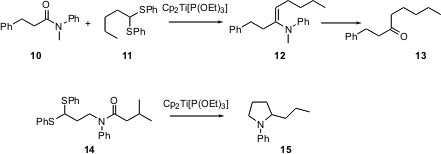
The Wittig reaction efficiently olefinates aldehydes and ketones, but not esters or amides. Several early-transition-metal approaches have been taken to this problem. Recently, Takeshi Takeda of the Tokyo University of Agriculture and Technology reported (Tetrahedron Lett. 2003, 44, 5571.DOI: 10.1016/S0040-4039(03)01385-6)that the titanocene reagent can effect the condensation of an amide 10 with a thioacetal 11 to give the enamine 12. On hydrolysis, 12 is converted into the ketone 13. When the reaction is intramolecular, reduction proceeds all the way, to give the pyrrolidine15.