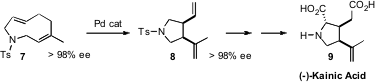(-)-Kainic Acid (Tomooka), Dasycarpidone (Bennasar), (-)-Cephalotaxine (Ishibashi)
and Lysergic Acid (Fujii/Ohno)
Intriguing strategies have been developed for the stereocontrolled assembly
of complex alkaloid structures. Brian M. Stoltz of Caltech prepared
(J. Formula of 4-Bromo-3,6-dichloropyridazine Am. Chem. Soc. 2008, 130, 13745
DOI: 10.1021/ja804738b)
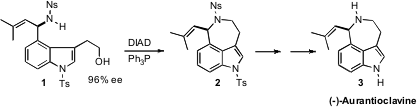
the enantiomerically-pure alcohol
precursor to the secondary amine 1 by enantioselective oxidation of the
racemic alcohol. Intramolecular
Mitsunobu coupling of 1 then led to (-)-Aurantioclavine
(3).
Yoshiaki Nakao and Tamejiro Hiyama of Kyoto University and Sensuke Ogoshi of
Osaka University developed
(J. Am. Chem. Soc. 2008, 130, 12874
DOI: 10.1021/ja805088r)
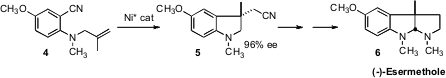
an enantioselective Ni catalyst for the cyclization of 4 to 5. PMID:25147652
Oxidation and cyclization then delivered (-)-Esermethole (6). 4-Chloropyridazin-3-ol Formula
Although the sulfonamide 7 appears to be prochiral, in fact its two
most stable conformations are bent, and enantiomers of each other, with a
significant barrier for interconversion. Katsuhiko Tomooka of Kyushu University separated
(Tetrahedron Lett. 2008, 49, 6327
DOI: 10.1016/j.tetlet.2008.08.058)
the enantiomers of 7, then carried the enantiomercially-pure 7 on, by
Pd-catalyzed Cope rearrangement, to 8 and so to (-)-Kainic Acid (9).
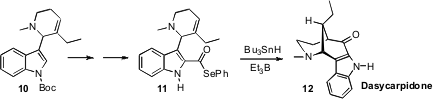
M.-Lluïsa Bennasar of the University of Barcelona prepared
(J. Org. Chem. 2008, 73, 9033
DOI: 10.1021/jo801998h)
the acyl selenide 11 from the indole 10.
While the radical derived from 11 might have been expected to undergo
5-exo cyclization, in the event the 6-endo mode dominated, to give Dasycarpidone
(12) and its diastereomer.
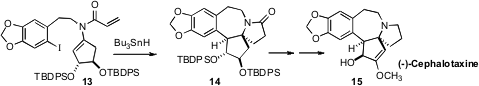
Hiroyuki Ishibashi of Kanazawa University showed
(Org. Lett. 2008, 10, 4129
DOI: 10.1021/ol801747m)
that the radical cascade cyclization of the enamine 13,
derived from diethyl tartrate, proceeded with remarkable diastereocontrol, to
give 14. The amide 14 was converted to (-)-Cephalotaxine (15).
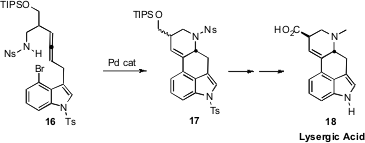
Nobutaka Fujii and Hiroaki Ohno, also of Kyoto University, used
(Org. Lett. 2008, 10, 5239
DOI: 10.1021/ol8022648)
a Pd catalyst to mediate the cascade cyclization
of 16 to 17. Although 16 has two stereogenic centers,
including the allene, it is the aminated stereogenic center of 17 that
sets the absolute configuration of the product Lysergic Acid (18). One
intermediate in the conversion of 16 to the tetracyclic 17 is the
tricyclic π-allyl Pd complex. If all the material could be channeled
through that pathway, there is a good chance that the chiral Trost
catalyst could effectively control the absolute configuration of the aminated
stereogenic center as it is formed, leading to the enantiomerically enriched
product 18.