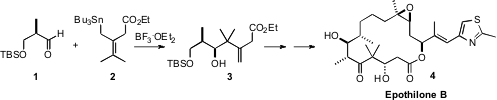
The total synthesis of Epothilone B (4), the first natural product (with
Epothilone A) to show the same microtubule-stabilizing activity as paclitaxel (Taxol®),
has attracted a great deal of attention since that activity was first reported
in 1995. Price of Sodium Iodide,99% The total synthesis of 4 devised
(J. Org. Chem. 2008, 73, 9675.
DOI: 10.1021/jo802215v)
by Gary E. Keck of the University of Utah was based in large
part on the stereoselective allyl stannane additions (e.g. 1 + 2 →
3) that his group originated.
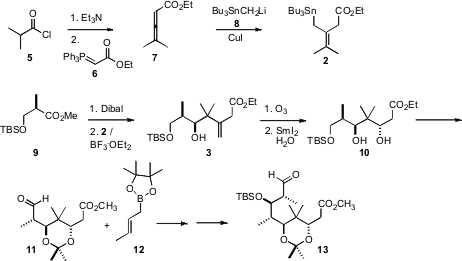
The allyl stannane 2 was prepared from the acid chloride 5. PMID:23829314 Ethyl 2-oxo-2-(2-oxocyclohexyl)acetate site
Exposure of 5 to Et3N generated the ketene, that was
homologated with the phosphorane 6 to give the allene ester 7.
Cu-mediated conjugate addition of the stannylmethyl anion 8 then
delivered 2.
The benzyloxy aldehyde 1 was prepared from the ester 9 by
reduction with Dibal. Felkin-controlled 1,2-addition of the allyl stannane 2
established the relative configuration of the secondary alcohol of 3,
that was then used to control the relative configuration of the new alcohol in
10. Addition of the crotyl borane 12 to the derived aldehyde 11
also proceeded with high diastereocontrol.
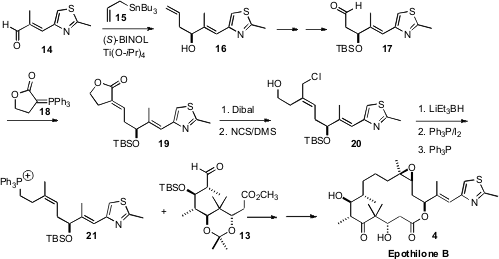
The other component of 4 was prepared from the aldehyde 14.
Enantioselective allylation, by the method the authors developed, delivered the
alcohol 16. The Z trisubstituted alkene was then assembled by
condensing the aldehyde 17 with the phosphorane 18. Dibal
reduction of the product lactone 19 gave a diol, the allylic alcohol of
which was selectively converted to the chloride with the
Corey-Kim reagent.
Hydride reduction then delivered the desired homoallylic alcohol, that was
converted to the phosphonium salt 21. Condensation of 21 with
13 gave the diene, that was carried on to Epothilone B (4).
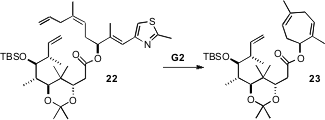
The synthesis of Epothilone B (4) as originally conceived by the
authors depended on ring-closing metathesis of the triene 22. They
prepared 22, but on
exposure to the second-generation Grubbs catalyst it
was converted only to 23. The authors concluded that the trans
acetonide kept 22 in a conformation that did not allow the desired macrocyclization.