Conjugate addition-enolate trapping, a strategy originally developed by
Gilbert Stork, has become a powerful method for stereocontrolled ring
construction. A key step in the synthesis of (-)-Clavirolide C (3) reported
(J. Am. Chem. PMID:23927631 Soc. 2008, 130, 12904
DOI: 10.1021/ja8058414)
by Amir H. VcMMAE custom synthesis Hoveyda of Boston College occurred early on, with the enantioselective conjugate
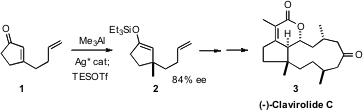
addition of Me3Al to 1 to give the silyl enol ether 2.
Enantioselective conjugate addition to establish a quaternary center β on a
cyclohexanone had been established (![]()
2008, August 18), but not yet on
cyclopentanones. Formula of 1361220-22-5 Professor Hoveyda found that a modified form of the Ag catalyst
that they had published earlier, in combination with the Lewis acidic AlMe3,
effected conjugate addition to 1 in 84% ee. Quenching of the reaction mixture
with triethylsilyl triflate led to the enol silyl ether 2.
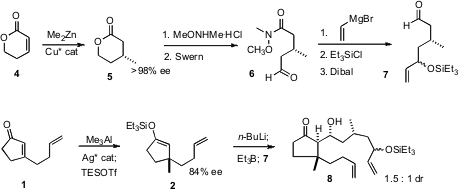
The assembly of the 11-membered ring of 3 also began with an enantioselective
conjugate methylation, of the lactone 4 with Me2Zn, again using a catalyst
developed by Professor Hoveyda. Opening of the lactone 5 followed by
Swern
oxidation gave the
Weinreb amide 6, that was homologated and reduced to give
7.
Addition of n-BuLi to 2 regenerated the enolate. There were two issues in the
addition of that enolate to the aldehyde 7: syn vs. anti stereocontrol, and
control of the configuration of the newly formed ternary center on the ring
relative to the already-established quaternary center. Inclusion of Et3B in the
reaction mixture assured anti aldol formation, but there was only a modest
preference for the desired bond formation trans to the slightly more bulky
butenyl group, to give 8.
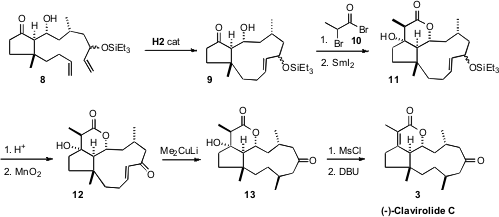
Medium rings are more strained than are larger rings. The diene 8 was reluctant to
close with the second generation Grubbs catalyst, but the catalyst
developed by Professor Hoveyda worked well. The δ-lactone of 3 was then
constructed by acylation of 9 with 10 followed by reductive cyclization with
SmI2. Conjugate addition to the derived enone 12 on the outside face of the
medium ring alkene gave the desired 13 (9:1 dr). This reaction may be proceeding
via the s-cis conformer, as the more stable s-trans conformer would have been
expected to give the other diastereomer. Dehydration of 13 then delivered (-)-Clavirolide
C (3).
This concise synthesis of the dolabellane 3 showcases the power of the
catalytic enantioselective methods for the construction of both ternary and
quaternary, including cyclic quaternary, centers that Professor Hoveyda has
developed. Clearly, asymmetric transformation of inexpensive prochiral ring
precursors such as 1 and 4 will make advanced, high ee intermediates such as
2
and 5 much more readily available than they have been in the past.