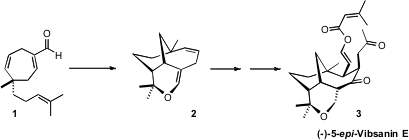
There are currently 61 known vibsane-type diterpenes, as exemplified by
(-)-5-epi-Vibsanin E (3). The first synthesis of 3, described
(J. Price of 79208-84-7 Am. Chem. Soc. 2009, 131, 8329.
DOI: 10.1021/ja9019484)
by Huw M. L. Amino-PEG3-C2-Amine web Davies of Emory University and Craig M. Williams of the University of
Queensland, was based on the enantioselective seven-membered ring construction
developed by the Davies group and the end game established by the Williams group.
A key step in the synthesis was the intramolecular hetero
Diels-Alder
cyclization of 1 to 2.
The absolute configuration of 1 was set by the Rh-mediated
cyclopropanation of 4 with the diazo ester 5. PMID:23577779 Though closely
related to the α-diazo β-keto ester 6, the alkene of 5 donates electron density
to the intermediate Rh carbene, making it more susceptible to the influence of
the chiral ligands. The alkene of the enol ether then participated in the Cope
rearrangement, delivering 8. Routine functional group transformation then
converted 8 to 1, that cyclized smoothly to 2.
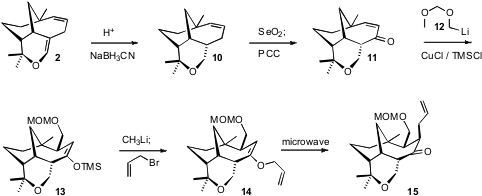
The enol ether of 2 was reduced with high diastereocontrol to give 10. The
ketone was installed by allylic oxidation, setting the stage for attachment of
the two pendant sidechains of 3 by conjugate addition followed by enolate
trapping. Cu-catalyzed addition of the α-oxygenated organolithium 12 proceeded
well in the presence of TMS-Cl, to establish the silyl enol ether 13. Allylation
of the regenerated enolate proceeded at oxygen, but the enol ether 14 so prepared rearranged to the desired C-alkylated product
15 on microwave heating.
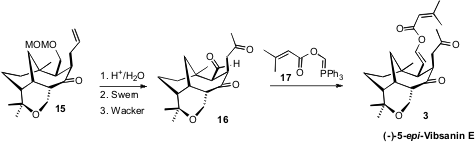
The synthesis endgame was based on an unusual transformation, the addition to
the keto aldehyde 16 of the phosphonium salt 17, developed
(Tetrahedron 2008, 64, 6482.
DOI: 10.1016/j.tet.2008.04.068)
by the Williams group. This allowed the introduction of the complete
vinyl ester array of (-)-5-epi-Vibsanin E (3).
This synthesis illustrates the power of the elegant enantioselective
seven-membered ring construction developed by the Davies group. The Williams
phosphonium salt will also have general applicability. In a simpler
manifestation, conversion of an aldehyde to, e.g., the enol benzoate, followed
by exposure to dilute methoxide, will allow the conversion of an aldehyde to the
aldehyde one carbon longer, without the acidic hydrolysis usually required for
such a transformation.