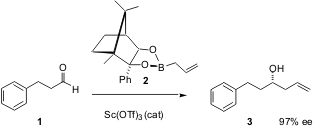
The enantioselective addition of allyl organometallics to carbonyls has become one of the workhorses of organic synthesis. PMID:24318587 Dennis Hall of the University of Alberta reports (J. Am. Chem. Soc. 2003, 125, 10160.DOI: 10.1021/ja036807j)the scandium triflate catalysis chiral allylboronic acids become more effective tools. Bis(3-aminopropyl) ether Data Sheet The best of these, the Hoffmann camphor derivative2, adds to aldehydes under Sc(OTf)3 catalysis with excellent enantiomeric excess. The reaction works equally well for methallyl, and for the E and Z crotyl boronic acids. The crotyl derivatives react with the expected high diastereocontrol. A limitation to the boronate additions is that branched chain aldehydes give low yields. 57595-23-0 supplier
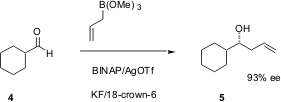
A complementary method for allylation reported (J. Org. Chem. 2003,68, 5593.DOI: 10.1021/jo020691c)by Hishashi Yamamoto, now of the University of Chicago, works particularly well with branched aldehydes. The allyl source is the inexpensive allyltrimethoxysilane, with BINAP-complexed Ag ion as the catalyst. Activation of the allylsilane with KF and 18-crown-6 is critical to the success of this reaction.
Thomas Lectka of Johns Hopkins University has reported (J. Org. Chem. 2003,68, 5819.DOI: 10.1021/jo034150e)that benzoylquinine (BQ) catalyzes the two-carbon homologation of a ketene, derived from the acid chloride, with chloroamide such7, to give the β-amino acid derivative 8 with control of both relative and absolute configuration. The authors suggest that the BQ is involved five times in the course of the transformation of 6 into 8. The two esters of the product are differentiated, so one can imagine, inter alia, reduction of one or the other to the alcohol, and formation of the activated aziridine orazetidine. These could then be further homologated.
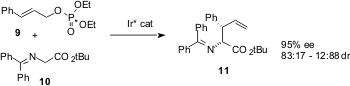
As reported (J. Org. Chem. 2003, 68, 6197.DOI: 10.1021/jo034638f)by Yoshjii Takemoto of Kyoto University, α-amino acids can be prepared in high enantiomeric and diastereomeric excess by Ir-mediated two-carbon homologation of allylic phosphates such as 9 with the protected glycine 10. Either diastereomer can be made dominant by varying the reaction conditions.