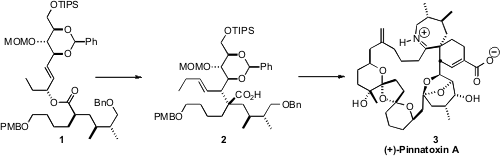
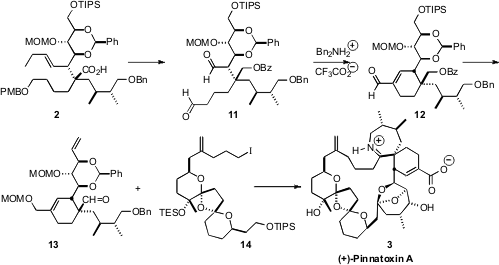
(+)-Pinnatoxin A (3), isolated from the shellfish Pinna muricata, is thought
to be a calcium channel activator. A key transformation in the synthesis of 3 reported
(J. Am. Chem. Soc. PMID:23554582 Formula of 6-Fluorobenzofuran-2-carboxylic acid 2008, 130, 3774.
DOI: 10.1021/ja800435j)
by Armen Zakarian, now at the University of California, Santa Barbara, was the
diastereoselective Claisen rearrangement of 1 to 2.
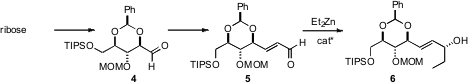
The alcohol portion of ester 1 was derived from the aldehyde 4,
prepared from D-ribose. The absolute configuration of the secondary allylic
alcohol was established by chiral amino alcohol catalyzed addition of diethyl
zinc to the unsaturated aldehyde 5. 3945-69-5 custom synthesis
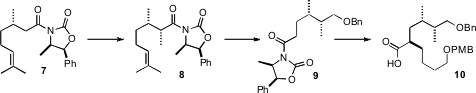
The acid portion of the ester 1 was prepared from (S)-citronellic
acid, by way of the Evans imide 7. Methylation proceeded with high
diasterocontrol, to give 8. Functional group manipulation provided the
imide 9. Alkylation then led to 10, again with high
diastereocontrol. In each case, care had to be taken in the further processing
of the α-chiral acyl oxazolidinones. Direct
NaBH4 reduction of
8 delivered the
primary alcohol. To prepare the acid 10, the alkylated acyl
oxazolidinone was hydrolyzed with alkaline
hydrogen peroxide.
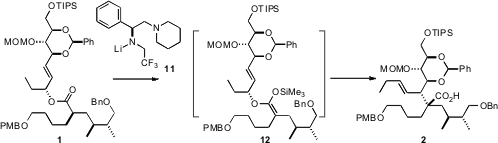
On exposure of the ester 1 to the enantiomerically-pure base 11,
rearrangement proceeded with high diastereocontrol, to give the acid 2. This
outcome suggests that deprotonation proceeded to give the single geometric form
of the enolate, that was then trapped to give specifically the ketene silyl
acetal 12. This elegant approach is dependent on both the ester 1 and the base
11 being enantiomerically pure.
The carbocyclic ring of pinnatoxin A (3) was assembled by intramolecular aldol
condensation of the dialdehyde 11. This outcome was remarkable, in that
11 is
readily epimerizable, and might also be susceptible to β-elimination. Note that
the while the diol corresponding to 11 could be readily oxidized to 11 under
Swern conditions, attempts to oxidize the corresponding hydroxy aldehyde were
not fruitful.