The tetracyclic alkaloids Nankakurine A and Nankakurine B were isolated from
the club moss Lycopodium hamiltonii. A preliminary study of the
biological activity of Nankakurine A suggested that it could induce secretion of
neurotrophic factors and promote neuronal differentiation. The key step in the
first syntheses of Nankakurine A and of Nankakurine B, reported
(J. PMID:23756629 Am. Imidazo[1,2-a]pyridine-8-carbaldehyde Data Sheet Chem. Soc. 2008, 130, 11297.
DOI: 10.1021/ja804624u)
by Larry E. Overman of the University
of California, Irvine was the intriguing intramolecular aza-Prins cyclization of
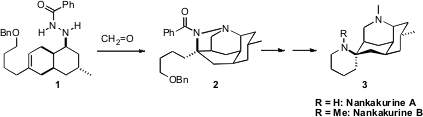
1 to 2.
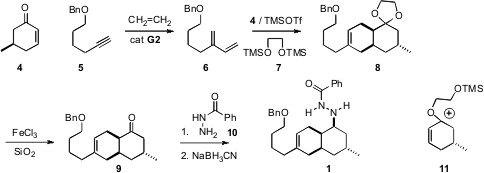
The starting material for the synthesis was 5-methyl
cyclohexenone 6,
prepared from (R)-pulegone. The diene 5 was prepared from the
alkyne 4, following the procedure developed by Diver. There were two
issues in developing the
Diels-Alder addition of the enone 4 to the diene
6. 131726-65-3 site The first was the relative lack of reactivity of 4 as a
dienophile. The other issue was the ready epimerization of the product ketone
9. Both of these problems were solved using the activation method devised by
Gassman. Condensation of 4 with 7 in the presence of the bis-silyl
ether 7 and the diene 6 at cryogenic temperatures led to the ketal
8. It is thought that the active dienophile was the cation 11.
Gentle hydrolysis of the ketal 8 was effected with minimal
epimerization. Reductive amination with the hydrazide 10 proceeded with
high diastereocontrol, to give the precursor 1.
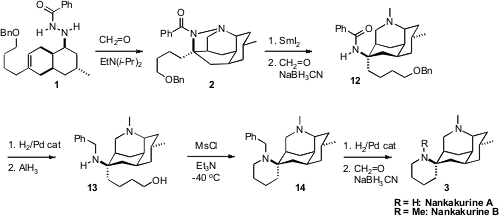
The intramolecular aza-Prins cyclization of 1 to 2 proceeded
well, though the desired tetracyclic 2 was only observed when base was
included in the reaction medium. In the absence of base,
tricyclic alkenes dominated.
Reduction of the N-N bond of 2 proceeded smoothly with freshly
prepared SmI2. After reductive methylation, hydrogenation removed the
benzyl ether, and AlH3 converted the benzamide to the benzyl amine.
At low temperature, mesylation of the alcohol was apparently faster than
mesylation of the secondary amine, enabling cyclization to 14. Removal of
the benzyl protecting group gave Nankakurine A, which was successfully
methylated to give Nankakurine B.
The completion of a total synthesis is an anxious moment, as for the first
time it is possible to compare spectra of the synthetic material with those
reported for the natural product. There is always a concern as to whether or not
the spectra are being acquired under precisely the same conditions employed by
those who did the initial isolation. This is particularly true for very polar
molecules such as these diamines. In fact, the spectra in CD3OD did
not initially match, but on the addition of small amounts of CF3CO2H
they were brought into congruence.
Although in this synthesis the starting enantiomerically-pure cyclohexenone
4 was derived from natural sources, one could imagine that
enantioselective conjugate methylation of cyclohexenone or a derivative could
get one into the same manifold.