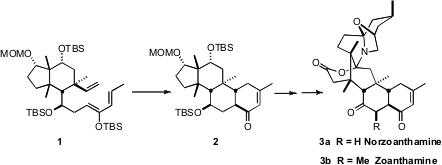
The Zoanthus alkaloids, exemplified by (-)-norzoanthamine (3a)
and zoanthamine (3b), show promising activity against osteoporosis. PMID:24189672
Susumu Kobayashi of the Tokyo University of Science assembled
(Angew. Chem. Int. Ed. 2009, 48, 1400,
DOI: 10.1002/anie.200804544;
Angew. Chem. Buy57595-23-0 Int. Ed. 2009, 48, 1404,
DOI: 10.1002/anie.200804546)
the challenging tricyclic core of 3a employing the
intramolecular Diels-Alder cyclization of 1 to 2. The
cyclopentane of 1 served as useful
scaffolding, even though it was cleaved en route to 3a.
The cyclohexane ring of 1 has five of its six positions substituted,
including three that are alkylated quaternary centers. The starting point for
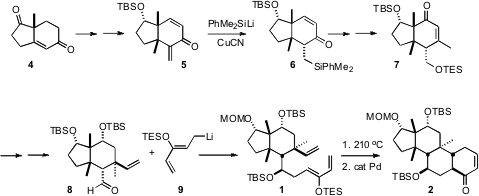
the preparation of 1 was the enantiomerically-pure Hajos-Parrish ketone
4, containing the first of the those quaternary centers. 6299-85-0 manufacturer Conjugate
addition of MeLi established the second quaternary center. The less stable endo
alkyl branch of 1 was installed by conjugate addition to the more
reactive α-methylene ketone of the cross-conjugated 5, followed by kinetic
quench. Addition of vinyl cuprate across the open face of the enone 7 then
established the final quaternary center, setting the stage for the
intramolecular Diels-Alder reaction. The silyl enol ether from the cyclization
of 1 was not stable, so it was directly oxidized to the enone 2.
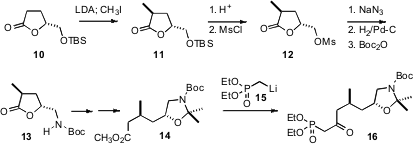
The keto phosphonate 16 for the last two rings of 3a was prepared from the
previously-reported crystalline glutamic acid-derived mesylate 12. Reduction and homologation delivered the ester
14, that was condensed with the phosphonate
anion 15 to give 16.
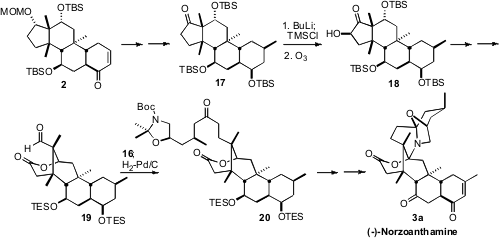
The congested
cyclopentanone 17, derived from 2, was most efficiently
deprotonated with n-BuLi. Exposure of the resulting silyl enol ether to ozone
led to the α-hydroxylated product 18. Unexpectedly but happily, oxidative
cleavage of 18 delivered, after deprotection and reprotection, the more
congested aldehyde 19. This cleavage may be proceeding by tautomerization of
18
to the regioisomeric keto alcohol. The aldehyde 19 was condensed with the keto
phosphonate 16, to give, after hydrogenation, the keto lactone 20. A series of
oxidation state adjustments then completed the synthesis of (-)-norzoanthamine (3a).
The preparation of 3a outlined here underlines the importance of developing
new methods for concise stereocontrolled
carbocyclic construction. The utility
of an enantiomerically-pure bicyclic scaffold such as 4 for subsequent relative
stereocontrol is particularly apparent.