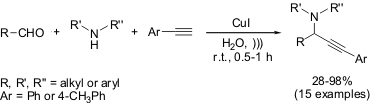
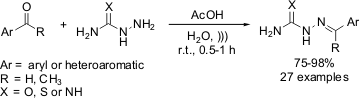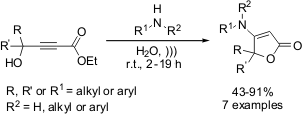High yielding ultrasound mediated three-component synthesis of substituted
propargylamines
Sreedhar and co-workers from Indian Institute of Chemical Technology have
described a mild one-pot, three-component synthesis of substituted
propargylamines by using ultrasound irradiation
(Tetrahedron Lett. 2005, 46, 7019.
DOI: 10.1016/j.tetlet.2005.08.047)
In this A3-coupling reaction, the sound waves promote a fast addition of metallic
acetylides to imides that have been generated in situ. The acoustic protocol has
been employed successfully for synthesizing 15 propargylamines in high yields. 2246363-82-4 Order
It has been found that 4- or 3-methoxybenzaldehyde substrates were weakly
reactive under such conditions, thus their use resulted in poor conversions. The
use of ultrasound irradiation has decreased the reaction time as well as the use
of large amount of copper catalyst in comparison to conventional procedures. 1240597-30-1 Formula
Moreover, in this method, commonly used organic solvents have been replaced by
water, thus it may be considered as an eco-friendly protocol. In the light of
these findings, He and co-workers have found that this protocol of an A3-coupling reaction also
works well under microwave irradiation
(Synth. PMID:23659187 Commun. 2007, 37, 849.
DOI: 10.1080/00397910601131320).
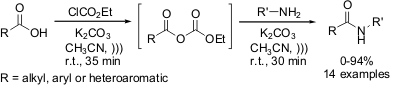
First high-yielding ultrasound-mediated synthesis of functionalized amides
Recently, the group of Srivastava from Federal University of Pernambuco,
Brazil, has reported a new clean, one-pot synthesis of secondary amides from
carboxylic acids and amines by employing ethyl chloroformate as coupling agent
and ultrasound irradiation.
(Ultrasonics Sonochem. 2009, 16, 737.
DOI: 10.1016/j.ultsonch.2009.04.006).
The protocol has been used to
synthesize fourteen amides in moderate to good yields in 1 h of total reaction
time. The reaction has worked well when employing aliphatic, aromatic as well as
heteroaromatic carboxylic acids. A large number of structurally different amines
have also been tested, and it was found that the reaction can be performed with either
alkylamines or anilines. Nonetheless, just one limitation of the presented
method has been found: the hindered adamantanamine did not undergo the reaction. The use of
sound wave activation has been found to be essential because when the reaction
was carried out by using conventional stirring, no changes in the starting
materials took place.
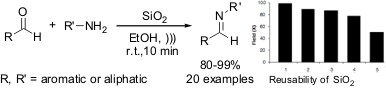
Ultrasound-mediated preparation of functionalized imines
Guzen and coworkers from State University of São Paulo (Brazil) have reported
a quick and cost-effective alternative for the synthesis of symmetrical and
unsymmetrical imines in high yields and E stereoselectivity, by using
unmodified silica as catalyst and ultrasound irradiation
(Tetrahedron Lett. 2007, 48, 1845.
DOI: 10.1016/j.tetlet.2007.01.014).
The advantageous properties of the silica rather than other solid catalysts are in
terms of yields, costs, reaction conditions, and also quantities of reactants.
The efficiency of this method was attributed to the ability of ultrasonic
irradiation to increase the available surface of silica, at the same time,
pushing up the solubility of reactants in ethanol. The scale-up of the method
has been investigated and it was found that even at 50 mmol scale the reactions
have worked well. Furthermore, silica-gel catalyst has been used many times with
remarkable results and only slight loss of catalytic activity. Therefore, the
abovementioned approach is quite attractive for the preparation of imines on
laboratory or industrial scale.
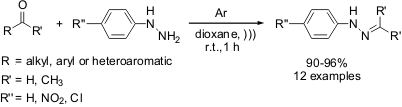
High yielding synthesis of aromatic hydrazones through ultrasonication
The arylhydrazone unit is widely used in pharmaceutical compositions and
consequently, the search for quick and mild syntheses of such important
compounds has been the focus of research in many research groups. In the light
of this scenario, Jarikote and co-workers from National Chemical Laboratory (India)
have developed a novel protocol for the catalyst-free synthesis of substituted
arylhydrazones by employing sonication as the energy source. (Ultrasonics
Sonochem. 2003, 10, 45.
DOI: 10.1016/S1350-4177(02)00100-1).
In fact, the reaction of arylhydrazines and aldehydes or ketones under
ultrasound irradiation at room temperature has been employed for synthesizing 12
products in high yields (90-96%) in just 1 h of reaction time. The method
presented a large scope of applications but, as a drawback, it demands the use
of an argon atmosphere, since low
conversion of starting materials took place without using such an inert gas.
High yielding preparation of aromatic thiosemicarbazones and analogues
Leite and co-workers from Federal University of Pernambuco (Brazil) have
described a rapid, diastereoselective (only E-isomers) and easy synthesis
of aromatic thiosemicarbazones using ultrasound irradiation at room temperature
(Tetrahedron Lett. 2008, 49, 1538.
DOI: 10.1016/j.tetlet.2007.12.103).
Although the reactants were insoluble in water,
ultrasound irradiation increased their solubility, thus an efficient and fast
condensation of carbonyl compounds with thiosemicarbazide was observed. Moreover,
this protocol has also been successfully applied for synthesizing aromatic
semicarbazones as well as guanyl hydrazones in good yields, while under
conventional heating, lower conversions are observed after 1 h of reaction time.
As a limitation, no conversion has been observed by using benzophenone as
substrate.
Hetero-Michael reactions of substituted aminofuran-2-one derivatives via
ultrasound irradiation
Recently, an interesting protocol for the hetero-Michael reactions to give
substituted aminofuran-2-one derivatives has been disclosed by Arcadi, Alfonsi
and Marinelli from University of L’Aquila-Via Vetoio (Italy). The reaction of
alkyl 4-hydroxy-2-alkynoates with primary or secondary amines under neutral
conditions and ultrasound irradiation at r.t. afforded highly functionalized
aminofuran-2-ones in yields ranging from 50-90%. Moreover, when these reactions were
performed with conventional stirring, lower conversions were always observed,
even under prolonged reaction times
(Tetrahedron Lett. 2009, 50, 2060.
DOI: 10.1016/j.tetlet.2009.02.106).