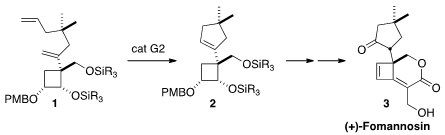
The compact sesquiterpene (+)-fomannosin (3), isolated from the pathogenic
fungus Fomes annonsus, presents an interesting set of challenges for the organic
synthesis chemist, ranging from the strained cyclobutene to the easily
epimerized cyclopentanone. PMID:24324376 In the synthesis of 3 developed
(J. Org. 4-Fluoropicolinaldehyde Chemscene Chem. 2008, 73, 4548.
DOI: 10.1021/jo8004233)
by Leo A. Paquette of Ohio State University, the cyclopentane was constructed by
ring-closing metathesis of 1. The real challenge of the synthesis was the
enantiospecific preparation of 1 from D-glucose.
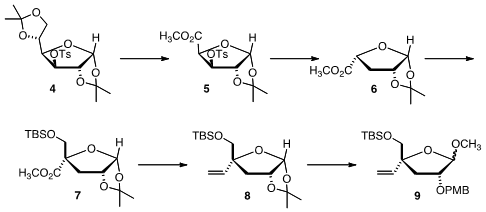
The starting point for the preparation of 1 was the glucose derivative
4. tert-Butyl 5-aminopentanoate supplier Selective acetonide hydrolysis followed by oxidative cleavage gave the
ester 5, which on base treatment followed by hydrogenation delivered the
endo ester 6. Condensation of the enolate of 6 with formaldehyde
proceeded with high diastereoselectivity, to give, after protection, the ester
7. Conversion of the ester to the vinyl group, exposure to methanolic
acid and ether formation completed the preparation of 9.
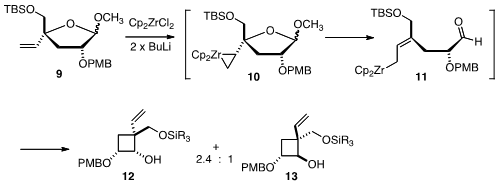
The construction of the cyclobutane of 1 was effected by an
interesting application of the Negishi reagent (Cp2ZrCl2/2
x BuLi). Complexation of Cp2Zr with the alkene followed by
elimination generated an allylic organometallic 11, which added to the
released aldehyde to give the
cyclobutanes 12 and 13 in a 2.4:1
diastereomeric ratio.
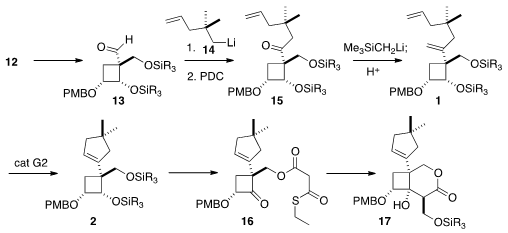
Homologation of the aldehyde 13 and subsequent oxidation were
straightforward, but subsequent methylenation of the hindered carbonyl was not.
At last, it was found that
Peterson olefination worked well.
Metathesis then
delivered the cyclopentene 2. The last carbons of the skeleton were added
by intramolecular aldol cyclization of the thioester 16.
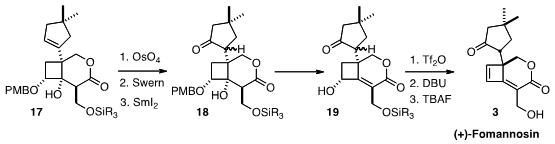
The seemingly simple task of converting the alkene of 17 into a ketone
proved challenging. Eventually, dihydroxylation followed by oxidation, and then
SmI2 reduction, completed the transformation. This still left the
challenge of controlling the cyclopentane stereogenic center. Remarkably,
dehydration and epimerization led to (+)-Fomannosin (3) as a single
dominant diastereomer.