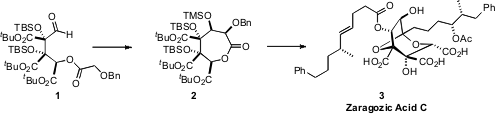
The zaragozic acids, exemplified by Zaragozic Acid C (3), are picomolar
inhibitors of cholesterol biosynthesis. Jeffrey S. PMID:26446225 2126818-91-3 custom synthesis Johnson of the University of
North Carolina developed
(J. Am. Chem. Soc. 98730-77-9 custom synthesis 2008, 130, 17281.
DOI: 10.1021/ja808347q)
an audacious silyl glyoxylate cascade approach to the oxygenated backbone
fragment 1. Intramolecular aldol cyclization converted 1 to 2, setting the stage
for the construction of 3.
The lactone 2 includes five stereogenic centers, two of which are quaternary.
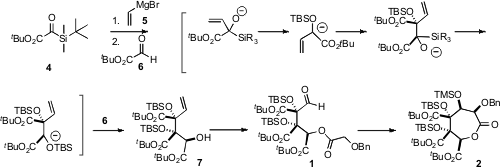
The authors were pleased to observe that exposure of 4 to vinyl magnesium
bromide (5) led, via condensation, silyl transfer, condensation, and again silyl
transfer, to a species that was trapped with t-butyl glyoxylate (6) to give
7 as a single diastereomer. This one step assembled three of the stereogenic centers of
2, including both of the quaternary centers. The alcohol 7 so prepared was
racemic, so the wrong enantiomer was separated by selective oxidation.
Intramolecular aldol reaction of the derived α-benzyloxy acetate 1 then
completed the construction of 2.
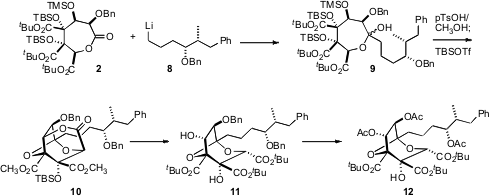
Addition of the alkyl lithium 8, again as a single enantiomerically-pure
diasteromer, to 2 gave the hemiketal 9. Exposure of 9 to acid initially gave a
mixture of products, but this could be induced to converge to the tricyclic
ester 10. To convert 10 to 11, the diastereomer that was needed for the
synthesis, two of the stereogenic centers had to be inverted. This was
accomplished by exposure to t-BuOK/t-amyl alcohol, followed by re-esterification.
The inversion of the secondary hydroxyl group was thought to proceed by
retro-aldol/re-aldol condensation.
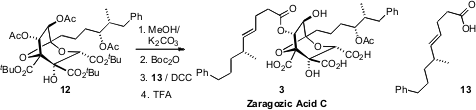
Debenzylation of 11 followed by acetylation delivered 12, an intermediate in
the Carreira synthesis of the zaragozic acids. Following that precedent, the
ring acetates of 12 were selectively removed, leaving the acetate on the side
chain. Boc protection was selective for the endo ring secondary hydroxyl,
leaving the exo ring secondary hydroxyl available for condensation with the
enantiomerically-pure acid 13. Global deprotection then completed the synthesis
of Zaragozic Acid C (3).
The key to the success of this synthesis of the complex spiroketal 3 was the
assembly of 7 in one step as a single diastereomer from the readily-available
building blocks 4, 5, and 6. This process, reminiscent of group transfer
polymerization, will be a useful complement to the cascade organocatalyzed aldol
condensations that have recently been developed.