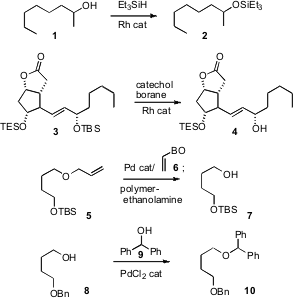
Several noteworthy new developments in the protection and deprotection of alcohols have been reported. Price of 2-Aminoacetamide Andrea Biffis of the Università di Padova has developed (Adv. Synth. Catal. 2007, 349, 2485.DOI: 10.1002/adsc.200700167)a Rh catalyst that effected silylation of alcohols such as 1 to the TES ether2 at just 0.01% loading. Joshua Rokach of the Florida Institute of Technology has observed (Tetrahedron Lett. 2007, 48, 5289.DOI: 10.1016/j.tetlet.2007.05.118)that the reverse reaction, Rh-catalyzed desilylation of 3, was highly selective for the less congested site, even removing the usually less reactiveTBS ether of 3 and leaving the more hindered TES ether. Hirokazu Tsukamoto of Tohoku University has devised (Tetrahedron Lett. PMID:23910527 5-Methoxyquinazolin-4(3H)-one uses 2007,48, 8438.DOI: 10.1016/j.tetlet.2007.10.001)an improved procedure for the deprotection of allyl ethers such as 5. Filtration of the reaction mixture through polymer-bound diethanolamine removed > 95% of the Pd from the product. Patrick Pale of the Université Louis Pasteur has established (Tetrahedron Lett. 2007, 48, 8895.DOI: 10.1016/j.tetlet.2007.10.045)improved conditions for preparing diphenylmethyl ethers such as 10. The protecting group was removed with the Pd catalyst and ethanol.
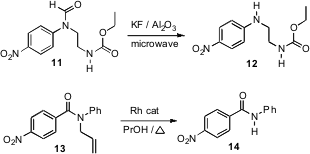
Amines can be activated for alkylation by N-formylation. Subsequent deformylation of the alkylated formamide 11 has been a challenge. Longqin Hu of Rutgers University has developed (Tetrahedron Lett. 2007, 48, 4585.DOI: 10.1016/j.tetlet.2007.04.127)microwave conditions that work well. Carbamates are stable, but esters are not. Protection of amides can also be important. Michael J. Zacuto of Merck Process in Rahway, NJ has optimized (J. Org. Chem. 2007, 72, 6298.DOI: 10.1021/jo070553t)the Rh-catalyzed deallylation of 13 to give 14.
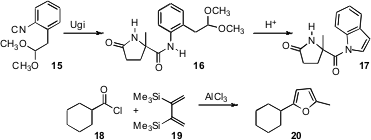
Carbonyl protection and deprotection is also important. Yoshihisa Kobayashi of the University of California, San Diego has devised (J. Org. Chem. 2007, 72, 3913.DOI: 10.1021/jo0700225)the isonitrile 15. Usually, the product 16 afterUgi condensation would be very difficult to hydrolyze. In the case of 16, mild acid effected cyclization to the acyl indole 17, which was easy to hydrolyze. In a different approach, Francesco Naso of the Università di Bari has shown (Chem. Commun. 2007, 3756.DOI: 10.1039/b705257j)that acid chlorides such as 18 condensed with 19 to give the furan 20. Such furans are easily oxidized, liberating the starting acid.
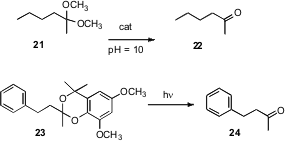
Acetals and ketals are usually removed with acid. Robert G. Bergman and Kenneth N. Raymond of the University of California, Berkeley have devised (Angew. Chem. Int. Ed. 2007, 46, 8587.DOI: 10.1002/anie.200703371)a self-assembling supramolecular catalyst that hydrolysed dimethyl acetals and ketals such as 21 in water at pH = 10. Pengfei Wang of the University of Alabama, Birmingham has designed (Org. Lett. 2007, 9, 2831.DOI: 10.1021/ol071085c)a protecting group 23 for aldehydes and ketones that was efficiently removed by photolysis.